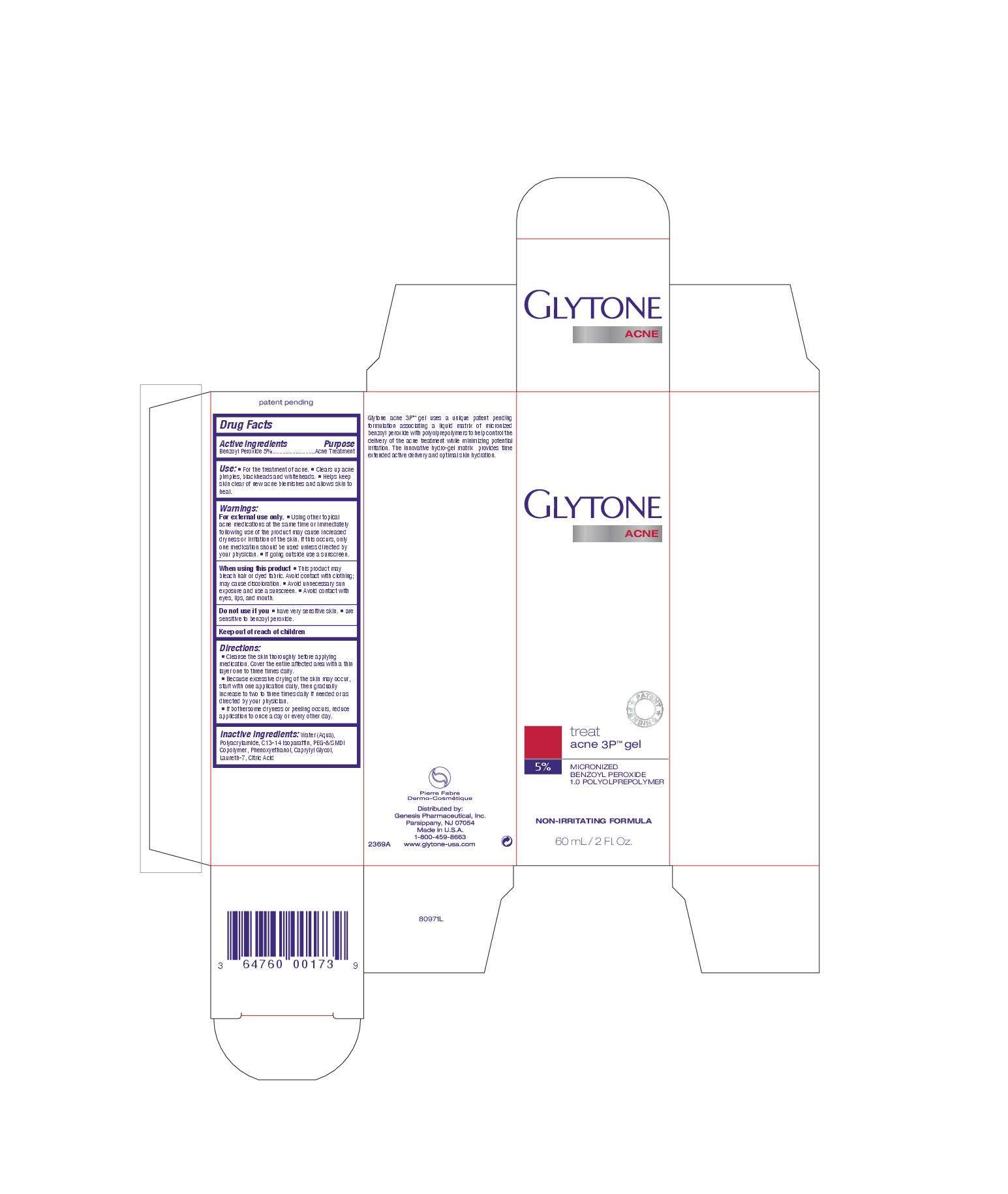
Glytone acne 3P
Genesis Pharmaceuticals
Genesis Pharmaceuticals
Glytone acne 3P
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients Purpose
Benzoyl Peroxide 5% Acne Treatment
Purpose
Acne treatment
Uses
Use:
For the treatment of acne
Clears up acne pimple, blackheads and whiteheads
Helps keep skin clear of new acne blemishes and allows skin to heal
Keep out of reach of children
Inactive ingredients: Water (aqua), polyacrylamide, C13-14 Isoparaffin, PEG-8/SMDI Copolyer, Phenoxyethanol, Caprylyl Glycol, Laureth-7, Citric acid
Directions:
Cleanse the skin thoroughly before applying medication. Cover the entire affected area with a thin layer one to three times daily
Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two to three times daily if needed or as directed by your physician
If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Glytone acne 3P uses a unique patent pending formulation associating a liquid matrix of micronized benzoyl peroxide with polyolprepolymers to help control the delivery of the acne treatment while minimizing potential irritation. The innovative hydro-gel matrix provides time extended active delivery and optimal skin hydration.
Glytone Acne
treat acne 3P gel
5% micronized benzoyl peroxide
1.0 polyolprepolymer
non-irritating formula
60 ml/2 fl. oz
Glytone acne 3Pbenzoyl peroxide GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||