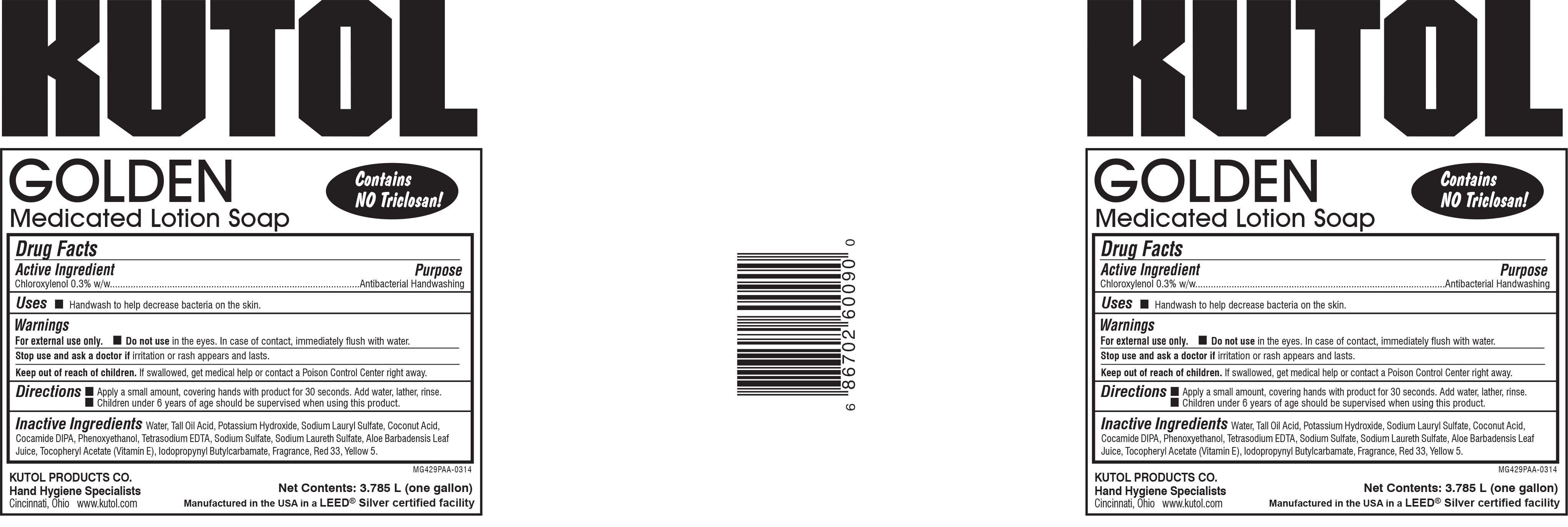
Golden Medicated
Golden Medicated
FULL PRESCRIBING INFORMATION
Active ingredient
Chloroxylenol 0.3% w/w.......Antibacterial Handwashing
Water, Tall Oil Acid, Potassium Hydroxide, Sodium Lauryl Sulfate, Coconut Acid, Cocamide DIPA, Phenoxyethanol, Tetrasodium EDTA, Sodium Sulfate, Sodium Laureth Sulfate, Aloe Barbadensis Leaf Juice, Tocopheryl Acetate (Vitamin E), Iodopropynyl Butylcarbamate, Fragrance, Red 33, Yellow 5.
Purpose
Handwash to help decrease bacteria on the skin.
For external use only.
Do not use in the eyes. In case of contact, immediately flush with water.
Stop use and ask a doctor if irritation or rash appears and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- Apply a small amount, covering hands with product for 30 seconds. Add water, lather and rinse.
- Children under 6 years of age should be supervised when using this product.
Uses
Handwash to help decrease bacteria on the skin.
Do not use in the eyes. In case of contact, immediately flush with water.
Stop use and ask a doctor if irritation or rash appears and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
 50865-060-09.jpg
50865-060-09.jpg
 50865-060-27.jpg
50865-060-27.jpg
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Golden MedicatedMedicated Lotion Soap SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||