Granisetron Hydrochloride
Granisetron hydrochloride tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- RX Only
- GRANISETRON HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- Clinical Trials
- INDICATIONS & USAGE
- GRANISETRON HYDROCHLORIDE CONTRAINDICATIONS
- PRECAUTIONS
- GRANISETRON HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- Granisetron Hydrochloride Tablets-20 Tablets
- Granisetron Hydrochloride Tablets-2 Tablets
FULL PRESCRIBING INFORMATION
RX Only
GRANISETRON HYDROCHLORIDE DESCRIPTION
endo18244
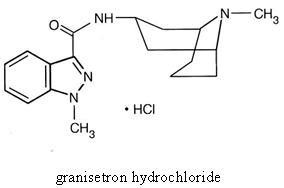
Tablets for Oral Administration
Each white, triangular, biconvex, film-coated Granisetron HCl Tablet contains 1.12 mg granisetron hydrochloride equivalent to granisetron, 1 mg. Inactive ingredients are: hyprmellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, sodium starch glycolate and titanium dioxide.
CLINICAL PHARMACOLOGY
Granisetron is a selective 5-hydroxytryptamine3 (5-HT3) receptor antagonist with little or no affinity for other serotonin receptors, including 5-HT1; 5-HT1A; 5-HT1B/C; 5-HT2; for alpha1-, alpha2-, or beta-adrenoreceptors; for dopamine-D2; or for histamine-H1; benzodiazepine; picrotoxin or opioid receptors.
333
Following single and multiple oral doses, Granisetron HCl Tablets slowed colonic transit in normal volunteers. However, Granisetron HCl had no effect on oro-cecal transit time in normal volunteers when given as a single intravenous (IV) infusion of 50 mcg/kg or 200 mcg/kg.
Pharmacokinetics
Table 1
Table 1 Pharmacokinetic Parameters (Median [range]). Following Granisetron HCl Tablets
|
*Not determined after oral administration; following a single intravenous dose of 40 mcg/kg, terminal phase half-life was determined to be 8.95 hours N.D. Not determined |
||||
|
|
Peak Plasma Concentration (ng/mL) |
Terminal Phase Plasma Half-Life (h) |
Volume of Distribution (L/kg) |
Total Clearance (L/h/kg) |
|
Cancer Patients 1 mg bid, 7 days (n=27) |
5.99 [0.63 to 30.9] |
N.D.*
|
N.D.
|
0.52 [0.09 to 7.37] |
|
Volunteers single 1 mg dose (n=39) |
3.63 [0.27 to 9.14] |
6.23 [0.96 to 19.9] |
3.94 [1.89 to 39.4] |
0.41 [0.11 to 24.6] |
Absorption
When Granisetron hydrochloride tablets were administered with food, AUC was decreased by 5% and Cmax increased by 30% in non-fasted healthy volunteers who received a single dose of 10 mg.
Distribution
Plasma protein binding is approximately 65% and granisetron distributes freely between plasma and red blood cells.
Metabolism
3
Elimination
Clearance is predominantly by hepatic metabolism. In normal volunteers, approximately 11% of the orally administered dose is eliminated unchanged in the urine in 48 hours. The remainder of the dose is excreted as metabolites, 48% in the urine and 38% in the feces.
Subpopulation
Gendermax
Elderly
The ranges of the pharmacokinetic parameters in elderly volunteers (mean age 71 years), given a single 40 mcg/kg intravenous dose of granisetron hydrochloride injection, were generally similar to those in younger healthy volunteers; mean values were lower for clearance and longer for half-life in the elderly.
Renal Failure PatientsTotal clearance of granisetron was not affected in patients with severe renal failure who received a single 40 mcg/kg intravenous dose of granisetron hydrochloride Injection.
Hepatically Impaired Patients:
Pediatric Patients:
Clinical Trials
Chemotherapy-Induced Nausea and vomiting
Granisetron HCl Tablets prevent nausea and vomiting associated with initial and repeat courses of emetogenic cancer therapy, as shown by 24-hour efficacy data from studies using both moderately- and highly-emetogenic chemotherapy.
Moderately Emetogenic Chemotherapy
22Table 2
| |
Percentages of Patients Granisetron HCl Tablet Dose |
|||
|
Efficacy Measures
|
0.25 mg bid (n=229) % |
0.5 mg bid (n=235) % |
1 mg bid (n=233) % |
2 mg bid (n=233) % |
Complete Response |
61 |
70 |
81  |
72 |
| No Vomiting |
66 |
77 |
88 |
79 |
| No Nausea |
48 |
57 |
63 |
54 |
Table 3
|
|
Percentages of Patients | ||
|
Efficacy Measures
|
Granisetron HCl tablets 1 mg bid (n = 354) % |
Granisetron HCl
tablets 2 mg qd (n = 343) % |
Prochlorperazine 10 mg bid (n=111) % |
Complete Response |
69 |
64 |
41
|
| No Vomiting
|
82 |
77 |
48
|
| No Nausea
|
51 |
53 |
35
|
Total Control |
51 |
50 |
33
|
Results from a Granisetron HCl Tablets 2 mg qd alone treatment arm in a third double-blind, randomized trial, were compared to prochlorperazine (PCPZ), 10 mg bid, derived from a historical control. The 24-hour results for Granisetron HCl Tablets 2 mg qd were statistically superior to PCPZ for all efficacy parameters: complete response (58%), no vomiting (79%), no nausea (51%), total control (49%). The PCPZ rates are shown in Table 3.
Cisplatin -Based Chemotherapy
The first double-blind trial compared Granisetron HCl Tablets 1 mg bid, relative to placebo (historical control), in 119 cancer patients receiving high-dose cisplatin (mean dose 80 mg/m2). At 24 hours, Granisetron HCl Tablets 1 mg bid was significantly (P<0.001) superior to placebo (historical control) in all efficacy parameters: complete response (52%), no vomiting (56%) and no nausea (45%). The placebo rates were 7%, 14%, and 7%, respectively, for the three efficacy parameters.
Results from a Granisetron HCl Tablets 2 mg qd alone treatment arm in a second double-blind, randomized trial, were compared to both Granisetron HCl Tablets 1 mg bid and placebo historical controls. The 24-hour results for Granisetron HCl Tablets 2 mg qd were: complete response (44%), no vomiting (58%), no nausea (46%), total control (40%). The efficacy of Granisetron HCl Tablets 2 mg qd was comparable to Granisetron HCl Tablets 1 mg bid and statistically superior to placebo. The placebo rates were 7%, 14%, 7%, and 7%, respectively, for the four parameters.
No controlled study comparing granisetron injection with the oral formulation to prevent chemotherapy-induced nausea and vomiting has been performed.
Radiation-Induced Nausea and Vomiting
Total Body Irradiation
3
Fractionated Abdominal Radiation
The efficacy of Granisetron HCl Tablets, 2 mg daily, was evaluated in a double-blind, placebo-controlled randomized trial of 260 patients. Granisetron HCl Tablets were given 1 hour before radiation, composed of up to 20 daily fractions of 180 to 300 cGy each. The exceptions were patients with seminoma or those receiving whole abdomen irradiation who initially received 150 cGy per fraction. Radiation was administered to the upper abdomen with a field size of at least 100 cm2.
The proportion of patients without emesis and those without nausea for Granisetron HCl Tablets, compared to placebo, was statistically significant (P<0.0001) at 24 hours after radiation, irrespective of the radiation dose. Granisetron HCl was superior to placebo in patients receiving up to 10 daily fractions of radiation, but was not superior to placebo in patients receiving 20 fractions.
Patients treated with Granisetron HCl Tablets (n=134) had a significantly longer time to the first episode of vomiting (35 days vs. 9 days, P<0.001) relative to those patients who received placebo (n=126), and a significantly longer time to the first episode of nausea (11 days vs. 1 day, P<0.001). Granisetron HCl provided significantly greater protection from nausea and vomiting than placebo.
INDICATIONS & USAGE
Granisetron HCl is indicated for the prevention of :
- Nausea and vomiting associated with initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin.
- Nausea and vomiting associated with radiation, including total body irradiation and fractionated abdominal radiation.
GRANISETRON HYDROCHLORIDE CONTRAINDICATIONS
Granisetron HCl is contraindicated in patients with known hypersensitivity to the drug or any of its components.
PRECAUTIONS
Granisetron HCl is not a drug that stimulates gastric or intestinal peristalsis. It should not be used instead of nasogastric suction. The use of Granisetron HCl in patients following abdominal surgery or in patients with chemotherapy-induced nausea and vomiting may mask a progressive ileus and/or gastric distention.
Drug Interactions
in vitroin vitro
in vitroin vivo
Carcinogenesis, Mutagenesis, Impairment Of Fertility
In a 24-month carcinogenicity study, rats were treated orally with granisetron 1, 5 or 50 mg/kg/day (6, 30 or 300 mg/m2/day). The 50mg/kg/day dose was reduced to 25 mg/kg/day (150 mg/m2/day) during week 59 due to toxicity. For a 50 kg person of average height (1.46 m2 body surface area), these doses represent 4, 20, and 101 times the recommended clinical dose (1.48 mg/m2, oral) on a body surface area basis. There was a statistically significant increase in the incidence of hepatocellular carcinomas and adenomas in males treated with 5 mg/kg/day (30 mg/m2/day, 20 times the recommended human dose based on body surface area) and above, and in females treated with 25 mg/kg/day (150 mg/m2/day, 101 times the recommended human dose based on body surface area). No increase in liver tumors was observed at a dose of 1 mg/kg/day (6 mg/m2/day, 4 times the recommended human dose based on body surface area) in males and 5 mg/kg/day (30 mg/m2/day, 20 times the recommended human dose based on body surface area) in females. In a 12-month oral toxicity study, treatment with granisetron 100 mg/kg/day (600 mg/m2/day, 405 times the recommended human dose based on body surface area) produced hepatocellular adenomas in male and female rats while no such tumors were found in the control rats. A 24-month mouse carcinogenicity study of granisetron did not show a statistically significant increase in tumor incidence, but the study was not conclusive.
Because of the tumor findings in rat studies, granisetron hydrochloride should be prescribed only at the dose and for the indication recommended (see INDICATIONS AND USAGE, and DOSAGE AND ADMINISTRATION).
Granisetron was not mutagenic in in vitro Ames test and mouse lymphoma cell forward mutation assay, and in vivo mouse micronucleus test and in vitro and ex vivo rat hepatocyte UDS assays. It, however, produced a significant increase in UDS in HeLa cells in vitro and a significant increased incidence of cells with polyploidy in an in vitro human lymphocyte chromosomal aberration test.
2Pregnancy
Pregnancy Category B.
22
Nursing Mothers
It is not known whether granisetron is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Granisetron HCl is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
During clinical trials, 325 patients 65 years of age or older received Granisetron HCl Tablets; 298 were 65 to 74 years of age, and 27 were 75 years of age or older. Efficacy and safety were maintained with increasing age.
GRANISETRON HYDROCHLORIDE ADVERSE REACTIONS
Chemotherapy-Induced Nausea and Vomiting
Over 3700 patients have received Granisetron HCl Tablets in clinical trials with emetogenic cancer therapies consisting primarily of cyclophosphamide or cisplatin regimens.
Table 4
Table 4 Principal Adverse Events in Clinical Trials
| |
Percent of Patients With Event
|
|||
| |
Granisetron HCl 1 mg bid (n=978) |
Granisetron HCl  tablets 2mg qd (n=1450) |
Comparator (n=599) |
Placebo (n=185) |
Headache |
21% |
20% |
13% |
12% |
| Constipation |
18% |
14% |
16% |
8% |
| Asthenia |
14% |
18% |
10% |
4% |
| Diarrhea |
8% |
9% |
10% |
4% |
| Abdominal pain |
6% |
4% |
6% |
3% |
| Dyspepsia |
4% |
6% |
5% |
4% |
Gastrointestinal:In single-day dosing studies in which adverse events were collected for 7 days, nausea (20%) and vomiting (12%) were recorded as adverse events after the 24-hour efficacy assessment period.
Hepatic:In comparative trials, elevation of AST and ALT (>2 times the upper limit of normal) following the administration of granisetron hydrochloride tablets occurred in 5% and 6% of patients, respectively. These frequencies were not significantly different from those seen with comparators (AST: 2%; ALT: 9%).
Cardiovascular:Hypertension (1%); hypotension, angina pectoris, atrial fibrillation, and syncope have been observed rarely.
Central Nervous System:Dizziness (5%), insomnia (5%), anxiety (2%), somnolence (1%). One case compatible with, but not diagnostic of, extrapyramidal symptoms have been reported in a patient treated with Granisetron HCl Tablets.
Hypersensitivity:Rare cases of hypersensitivity reactions, sometimes severe (eg, anaphylaxis, shortness of breath, hypotension, urticaria) have been reported.
Other:Fever (5%). Events often associated with chemotherapy also have been reported: leukopenia(9%), decreased appetite (6%), anemia (4%), alopecia (3%), thrombocytopenia (2%).
Over 5000 patients have received injectable granisetron hydrochloride in clinical trials.
| |
Percent of Patients with Event
|
|
| |
Granisetron hydrochloride 40 mcg/kg (n=1268) |
Comparator (n=422) |
| Headache |
14% |
6% |
| Asthenia |
5% |
6% |
| Somnolence |
4% |
15% |
| Diarrhea |
4% |
6% |
| Constipation |
3% |
3% |
Radiation-Induced Nausea and Vomiting
In controlled clinical trials, the adverse events reported by patients receiving Granisetron HCl Tablets and concurrent radiation were similar to those reported by patients receiving Granisetron HCl Tablets prior to chemotherapy. The most frequently reported adverse events were diarrhea, asthenia, and constipation. Headache, however, was less prevalent in this patient population.
OVERDOSAGE
There is no specific treatment for granisetron hydrochloride overdosage. In case of overdosage, symptomatic treatment should be given.
Overdosage of up to 38.5 mg of granisetron hydrochloride injection has been reported without symptoms or only the occurrence of a slight headache.
DOSAGE & ADMINISTRATION
Emetogenic Chemotherapy
Use in the Elderly, Pediatric Patients, Renal Failure Patients or Hepatically Impaired Patients
No dosage adjustment is recommended (see CLINICAL PHARMACOLOGY: Pharmacokinetics ).
Radiation (Either Total Body Irradiation or Fractionated Abdominal Radiation)
There is no experience with oral Granisetron HCl in the prevention of radiation-induced nausea and vomiting in pediatric patients.
Use in the Elderly
HOW SUPPLIED
NDC 67877-184-20-20’s pack
NDC 67877-184-02-2’s pack
Montvale, NJ07645.
Granisetron Hydrochloride Tablets-20 Tablets
RX Only
NDC67877-184-20
Each tablet contains 1.12 mg of granisetron Hydrochloride equivalent to granisetron, 1 mg.
This unit-dose package is not-child-resistant. For institutional use only.
Dosage: Chemotherapy: 2 mg once daily or 1mg twice daily. In the 2 mg once daily regimen, two 1 mg tablets are given up to 1 hour before chemotherapy. In the 1 mg twice daily regimen, the first 1 mg tablet is given up to 1 hour before chemotherapy and the second tablet 12 hours after the first.
Radiation: Two 1mg tablets are taken within 1 hour of radiation. See accompanying prescribing information.
Store between 20º and 25ºC (68º and 77ºF) [see USP Controlled Room Temperature]. Protect from light. Retain in carton until time of use.
M.L.: 164/MN/AP/95/F/R
Mfd for: Ascend Laboratories,
Montvale, NJ07645.
Mfd by: Natco Pharma Limited,
Kothur - 509 228, AP, India.

Blister Art Work
Granisetron Hydrochloride Tablets-2 Tablets
RX Only
NDC67877-184-02
Each tablet contains 1.12 mg of granisetron Hydrochloride equivalent to granisetron, 1 mg.
This unit-dose package is not-child-resistant. For institutional use only.
Dosage: Chemotherapy: 2 mg once daily or 1mg twice daily. In the 2 mg once daily regimen, two 1 mg tablets are given up to 1 hour before chemotherapy. In the 1 mg twice daily regimen, the first 1 mg tablet is given up to 1 hour before chemotherapy and the second tablet 12 hours after the first.
Radiation: Two 1mg tablets are taken within 1 hour of radiation. See accompanying prescribing information.
Store between 20º and 25ºC (68º and 77ºF) [See USP Controlled Room Temperature]. Protect from light. Retain in carton until time of use.
M.L.: 164/MN/AP/95/F/R
Mfd for: Ascend Laboratories,
Montvale, NJ07645.
Mfd by: Natco Pharma Limited,

Blister art work

Granisetron HydrochlorideGranisetron Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||