GRANISETRON HYDROCHLORIDE
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use granisetron hydrochloride injection safely and effectively. See full prescribing information for granisetron hydrochloride injection. Granisetron Hydrochloride Injection, USP, for intravenous use Initial U.S. Approval: 1993INDICATIONS AND USAGE3 Prevention of nausea and/or vomiting associated with initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin. (1) DOSAGE AND ADMINISTRATION Prevention of chemotherapy-induced nausea and vomiting (2.1): Recommended dosage is 10 mcg/kg intravenously within 30 minutes before initiation of chemotherapy Pediatric patients (2 to 16 years): Recommended dosage is 10 mcg/kg DOSAGE FORMS AND STRENGTHS Injection 1 mg/mL (free base). (3) CONTRAINDICATIONS Hypersensitivity to granisetron or to any of its components. (4) WARNINGS AND PRECAUTIONS Granisetron hydrochloride injection does not stimulate gastric or intestinal peristalsis and should not be used instead of nasogastric suction. (5.1) QT prolongation has been reported with granisetron hydrochloride injection. Use with caution in patients with pre-existing arrhythmias or cardiac conduction disorders. (5.2) Hypersensitivity reactions, such as anaphylaxis, shortness of breath, hypotension, and urticaria, may occur in patients with known hypersensitivity to other selective 5-HT3 receptor antagonists. (5.3) Side Effects Chemotherapy-induced nausea and vomiting (≥3%): Headache, and constipation (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Wockhardt USA LLC. at 1-800-346-6854 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Granisetron hydrochloride injection has been administered safely with benzodiazepines, neuroleptics, and anti-ulcer medications. (7) Does not appear to interact with emetogenic cancer chemotherapies. (7) Inducers or inhibitors of CYP450 enzymes may change the clearance and therefore the half-life of granisetron. (7) Coadministration of granisetron hydrochloride injection with drugs known to prolong the QT interval and/or are arrhythmogenic may result in clinical consequences. (7) USE IN SPECIFIC POPULATIONS Pregnancy: Use only if clearly needed. (8.1) Nursing mothers: Caution should be exercised when administered to a nursing woman. (8.3) Geriatric use: No differences in responses between the elderly and younger patients were observed in reported clinical experience. (8.5)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 GRANISETRON HYDROCHLORIDE INDICATIONS AND USAGE
- 2 GRANISETRON HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 GRANISETRON HYDROCHLORIDE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 GRANISETRON HYDROCHLORIDE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 GRANISETRON HYDROCHLORIDE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
3- The prevention of nausea and/or vomiting associated with initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin.
2 DOSAGE AND ADMINISTRATION
2.1 Prevention of Chemotherapy-Induced Nausea and Vomiting
Adult PatientsInfusion Preparation
Stability
Pediatric Patients
[see Clinical Studies (14)].
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Gastric or Intestinal Peristalsis
5.2 Cardiovascular Events
5.3 Hypersensitivity Reactions
36 ADVERSE REACTIONS
[see Warnings and Precautions (5.2) and Drug Interactions (7)].
6.1 Clinical Trials Experience
Chemotherapy-Induced Nausea and Vomiting
| Percent of Patients With Reaction | ||
|---|---|---|
| Granisetron Hydrochloride Injection 40 mcg/kg (n=1268) |
Comparator1
(n=422) |
|
| Headache | 14% | 6% |
| Constipation | 3% | 3% |
Hepatic:
Cardiovascular:
Central Nervous System:
Hypersensitivity:
Other:
6.2 Postmarketing Experience
[see Warnings and Precautions (5.2) and Drug Interactions (7)].
7 DRUG INTERACTIONS
in vitroin vitroin vitroin vivo
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B22
8.3 Nursing Mothers
It is not known whether granisetron is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when granisetron hydrochloride injection is administered to a nursing woman.
8.4 Pediatric Use
Chemotherapy-Induced Nausea and Vomiting
[See Dosage and Administration (2)] for use in chemotherapy-induced nausea and vomiting in pediatric patients 2 to 16 years of age. Safety and effectiveness in pediatric patients under 2 years of age have not been established.
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
3endo18244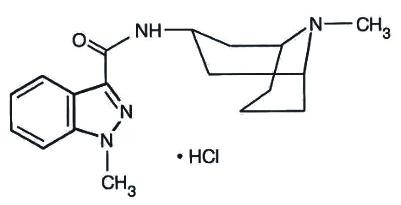
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
3311A1B/C21221333
12.3 Pharmacokinetics
Chemotherapy-Induced Nausea and Vomiting| Peak Plasma Concentration (ng/mL) |
Terminal Phase Plasma Half-Life (h) |
Total Clearance (L/h/kg) |
Volume of Distribution (L/kg) |
|
|---|---|---|---|---|
| Cancer Patients | ||||
| Mean | 63.8* | 8.95* | 0.38* | 3.07* |
| Range | 18.0 to 176 | 0.90 to 31.1 | 0.14 to 1.54 | 0.85 to 10.4 |
| Volunteers | ||||
| 21 to 42 years | ||||
| Mean | 64.3† | 4.91† | 0.79† | 3.04† |
| Range | 11.2 to 182 | 0.88 to 15.2 | 0.20 to 2.56 | 1.68 to 6.13 |
| 65 to 81 years | ||||
| Mean | 57.0† | 7.69† | 0.44† | 3.97† |
| Range | 14.6 to 153 | 2.65 to 17.7 | 0.17 to 1.06 | 1.75 to 7.01 |
†
Distribution
Metabolism
In vitro3
Elimination
Subpopulations
Gender
max
Elderly
Pediatric Patients
Renal Failure Patients
Hepatically Impaired Patients
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
222222222[see Indications and Usage (1) and Dosage and Administration (2)].
in vitroin vivoin vitroex vivoin vitroin vitro
2
14 CLINICAL STUDIES
14.1 Chemotherapy-Induced Nausea and Vomiting
Single-Day ChemotherapyCisplatin-Based Chemotherapy
| Granisetron Hydrochloride Injection | Placebo | P-Value | |
|---|---|---|---|
| Number of Patients | 14 | 14 | |
| Response Over 24 Hours | |||
| Complete Response2 | 93% | 7% | <0.001 |
| No Vomiting | 93% | 14% | <0.001 |
| No More Than Mild Nausea | 93% | 7% | <0.001 |
2
2
| Granisetron Hydrochloride Injection (mcg/kg) |
P-Value (vs. 2 mcg/kg) |
||||
|---|---|---|---|---|---|
| 2 | 10 | 40 | 10 | 40 | |
| Number of Patients | 52 | 52 | 53 | ||
| Response Over 24 Hours | |||||
| Complete Response2 | 31% | 62% | 68% | <0.002 | <0.001 |
| No Vomiting | 38% | 65% | 74% | <0.001 | <0.001 |
| No More Than Mild Nausea | 58% | 75% | 79% | NS | 0.007 |
2
22
| Granisetron Hydrochloride Injection (mcg/kg) |
P-Value (vs. 5 mcg/kg) |
||||||
|---|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 40 | 10 | 20 | 40 | |
| High-Dose Cisplatin | |||||||
| Number of Patients | 40 | 49 | 48 | 47 | |||
| Response Over 24 Hours | |||||||
| Complete Response2 | 18% | 41% | 40% | 47% | 0.018 | 0.025 | 0.004 |
| No Vomiting | 28% | 47% | 44% | 53% | NS | NS | 0.016 |
| No Nausea | 15% | 35% | 38% | 43% | 0.036 | 0.019 | 0.005 |
| Low-Dose Cisplatin | |||||||
| Number of Patients | 42 | 41 | 40 | 46 | |||
| Response Over 24 Hours | |||||||
| Complete Response2 | 29% | 56% | 58% | 41% | 0.012 | 0.009 | NS |
| No Vomiting | 36% | 63% | 65% | 43% | 0.012 | 0.008 | NS |
| No Nausea | 29% | 56% | 38% | 33% | 0.012 | NS | NS |
2
Moderately Emetogenic Chemotherapy
222
| Granisetron Hydrochloride Injection | Chlorpromazine1 | P-Value | |
|---|---|---|---|
| Number of Patients | 133 | 133 | |
| Response Over 24 Hours | |||
| Complete Response2 | 68% | 47% | <0.001 |
| No Vomiting | 73% | 53% | <0.001 |
| No More Than Mild Nausea | 77% | 59% | <0.001 |
2
Repeat-Cycle Chemotherapy
Pediatric Studies
2222
| Granisetron Hydrochloride Injection Dose (mcg/kg) | |||
|---|---|---|---|
| 10 | 20 | 40 | |
| Number of Patients | 29 | 26 | 25 |
| Median Number of Vomiting Episodes | 2 | 3 | 1 |
| Complete Response Over 24 Hours1 | 21% | 31% | 32% |
2
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
3
Manufactured by:
Distributed by:


GRANISETRON HYDROCHLORIDEGRANISETRON HYDROCHLORIDE INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!