Granisetron Hydrochloride
Breckenridge Pharmaceutical, Inc.
Natco Pharma Limited
GRANISETRON HYDROCHLORIDE TABLETS, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- RX Only
- GRANISETRON HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL TRIALS
- GRANISETRON HYDROCHLORIDE INDICATIONS AND USAGE
- GRANISETRON HYDROCHLORIDE CONTRAINDICATIONS
- PRECAUTIONS
- GRANISETRON HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- GRANISETRON HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 1.12 mg Tablet Carton
FULL PRESCRIBING INFORMATION
RX Only
GRANISETRON HYDROCHLORIDE DESCRIPTION
endo18244
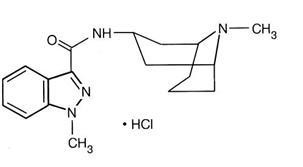
granisetron hydrochloride
Tablets for Oral Administration
Each white, triangular, biconvex, film-coated granisetron hydrochloride tablet contains 1.12 mg granisetron hydrochloride,USP equivalent to granisetron 1 mg.Inactive ingredients are: hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, sodium starch glycolate and titanium dioxide.
CLINICAL PHARMACOLOGY
3311A1B/C21221
333
Pharmacokinetics
Table 1.
Table 1 Pharmacokinetic Parameters (Median [range]) Following Granisetron Hydrochloride Tablets
| N.D. Not determined. * Not determined after oral administration; following a single intravenous dose of 40 mcg/kg, terminal phase half-life was determined to be 8.95 hours. |
||||
|
|
Peak Plasma Concentration (ng/mL) |
Terminal Phase Plasma Half-Life (h) |
Volume of Distribution (L/kg) |
Total Clearance (L/h/kg) |
|
Cancer Patients 1 mg bid, 7 days (n=27) |
5.99 [0.63 to 30.9] |
N.D.*
|
N.D.
|
0.52 [0.09 to 7.37] |
|
Volunteers single 1 mg dose (n=39) |
3.63 [0.27 to 9.14] |
6.23 [0.96 to 19.9] |
3.94 [1.89 to 39.4] |
0.41 [0.11 to 24.6] |
max
In vitro3
Gender
max
Elderly
Renal Failure Patients
Hepatically Impaired Patients
Pediatric Patients
CLINICAL TRIALS
Chemotherapy-Induced Nausea and Vomiting
Moderately Emetogenic Chemotherapy
22Table 2
Table 2 Prevention of Nausea and Vomiting 24 Hours Post-Chemotherapy*
|
*Chemotherapy included oral and injectable cyclophosphamide, carboplatin, cisplatin (20 mg/m2 to 50 mg/m2), dacarbazine, doxorubicin, epirubicin. † No vomiting, no moderate or severe nausea, no rescue medication. ‡ Statistically significant (P<0.01) vs. 0.25 mg bid. § Statistically significant (P<0.01) vs. 0.5 mg bid. |
||||
| |
Percentages of Patients Granisetron hydrochloride Tablet Dose |
|||
|
Efficacy Measures |
0.25 mg twice a day (n=229) % |
0.5 mg twice a day (n=235) % |
1 mg twice a day (n=233) % |
2 mg twice a day (n=233) % |
|
Complete Response† |
61 |
70‡
|
81‡§
|
72‡
|
| No Vomiting |
66 |
77‡
|
88‡
|
79‡
|
| No Nausea |
48 |
57 |
63‡
|
54 |
Table 3
Table 3 Prevention of Nausea and Vomiting 24 Hours Post-Chemotherapy*
|
* Moderately emetogenic chemotherapeutic agents included cisplatin (20 mg/m2 to 50 mg/m2), oral and intravenous cyclophosphamide, carboplatin, dacarbazine, doxorubicin. †Historical control from a previous double-blind granisetron hydrochloride trial. ‡ No vomiting, no moderate or severe nausea, no rescue medication. ¶ No vomiting, no nausea, no rescue medication. § Statistically significant (P<0.05) vs. prochlorperazine historical control. |
|||
| |
Percentage of Patients
|
||
|
Efficacy Measures
|
Granisetron
Hydrochloride 1 mg twice a day (n = 354) % |
Granisetron Hydrochloride Tablets
2 mg once a day (n = 343) % |
Prochlorperazine†
10 mg twice a day (n=111) % |
| Complete Response‡ |
69 §
|
64 §
|
41 |
| No Vomiting |
82 §
|
77 §
|
48 |
| No Nausea |
51 §
|
53 §
|
35 |
| Total Control¶
|
51 §
|
50 §
|
33 |
Table 3.
Cisplatin-Based Chemotherapy
2
Radiation-Induced Nausea and Vomiting
Total Body Irradiation
3
Fractionated Abdominal Radiation
2
GRANISETRON HYDROCHLORIDE INDICATIONS AND USAGE
- Nausea and vomiting associated with initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin.
- Nausea and vomiting associated with radiation, including total body irradiation and fractionated abdominal radiation.
GRANISETRON HYDROCHLORIDE CONTRAINDICATIONS
PRECAUTIONS
Drug Interactions
in vitroin vitro
in vitroin vivo
Carcinogenesis & Mutagenesis & Impairment Of Fertility
222222222
INDICATIONS AND USAGE DOSAGE AND ADMINISTRATION
in vitroin vivoin vitroex vivoin vitroin vitro
2
Pregnancy
Pregnancy Category B
22
Nursing Mothers
Pediatric Use
Geriatric Use
GRANISETRON HYDROCHLORIDE ADVERSE REACTIONS
PRECAUTIONS Drug Interactions).
Chemotherapy-Induced Nausea and Vomiting
Table 4.
Table 4 Principal Adverse Events in Clinical Trials
|
* Adverse events were recorded for 7 days when granisetron hydrochloride tablets were given on a single day and for up to 28 days when granisetron hydrochloride tablets were administered for 7 or 14 days. † Metoclopramide/dexamethasone; phenothiazines/dexamethasone; dexamethasone alone; prochlorperazine. |
||||
| |
Percent of Patients With Event
|
|||
|
Granisetron Hydrochloride Tablets*
1 mg twice a day (n=978) |
Granisetron Hydrochloride Tablets*
2 mg once a day (n=1450) |
Comparator†
(n=599) |
Placebo
(n=185) |
|
| Headache |
21% |
20% |
13% |
12% |
| Constipation |
18% |
14% |
16% |
8% |
| Asthenia |
14% |
18% |
10% |
4% |
| Diarrhea |
8% |
9% |
10% |
4% |
| Abdominal pain |
6% |
4% |
6% |
3% |
| Dyspepsia |
4% |
6% |
5% |
4% |
Gastrointestinal:
Hepatic:
Cardiovascular:
Central Nervous System:
Hypersensitivity:
Other:
Table 5
Table 5 Principal Adverse Events in Clinical Trials — Single-Day Chemotherapy
|
*Adverse events were generally recorded over 7 days post-granisetron hydrochloride Injection administration. † Metoclopramide/dexamethasone and phenothiazine/dexamethasone. |
||
| |
Percent of Patients with Event
|
|
|
Granisetron
hydrochloride Injection* 40 mcg/kg (n=1268) |
Comparator†
(n=422) |
|
| Headache |
14% |
6% |
| Asthenia |
5% |
6% |
| Somnolence |
4% |
15% |
| Diarrhea |
4% |
6% |
| Constipation |
3% |
3% |
Radiation-Induced Nausea and Vomiting
Postmarketing Experience
PRECAUTIONS Drug Interactions
OVERDOSAGE
GRANISETRON HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Emetogenic Chemotherapy
Use in the Elderly, Renal Failure Patients or Hepatically Impaired Patients
CLINICAL PHARMACOLOGY: Pharmacokinetics
Pediatric Use
Radiation (Either Total Body Irradiation or Fractionated Abdominal Radiation)
Pediatric Use
Use in the Elderly
HOW SUPPLIED
PRINCIPAL DISPLAY PANEL - 1.12 mg Tablet Carton
NDC 51991-735-20
Breckenridge
Pharmaceutical, Inc.
20 (2×10) Unit Dose Tablets
Granisetron Hydrochloride Tablets, USP
1 mg*
*Each tablet contains 1.12 mg granisetron hydrochloride USP
equivalent to granisetron, 1 mg
This unit-dose package is not-child-resistant.
For institutional use only.
Rx Only

Granisetron HydrochlorideGranisetron Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||