GUNA-Beta-ESTRADIOL
GUNA®-Beta-ESTRADIOL
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1. GUNA-BETA-ESTRADIOL INDICATIONS AND USAGE
- 2. GUNA-BETA-ESTRADIOL DOSAGE AND ADMINISTRATION
- 3. DOSAGE FORMS AND STRENGTHS
- 4. GUNA-BETA-ESTRADIOL CONTRAINDICATIONS
- 5. WARNINGS AND PRECAUTIONS
- 6. GUNA-BETA-ESTRADIOL ADVERSE REACTIONS
- 7. DRUG INTERACTIONS
- 8. USE IN SPECIFIC POPULATIONS
- 9. DRUG ABUSE AND DEPENDENCE
- 10. OVERDOSAGE
- 11. GUNA-BETA-ESTRADIOL DESCRIPTION
- 12. CLINICAL PHARMACOLOGY
- 13. NONCLINICAL TOXICOLOGY
- 14. CLINICAL STUDIES
- 15. HOW SUPPLIED/STORAGE AND HANDLING
- 16. PATIENT COUNSELING INFORMATION
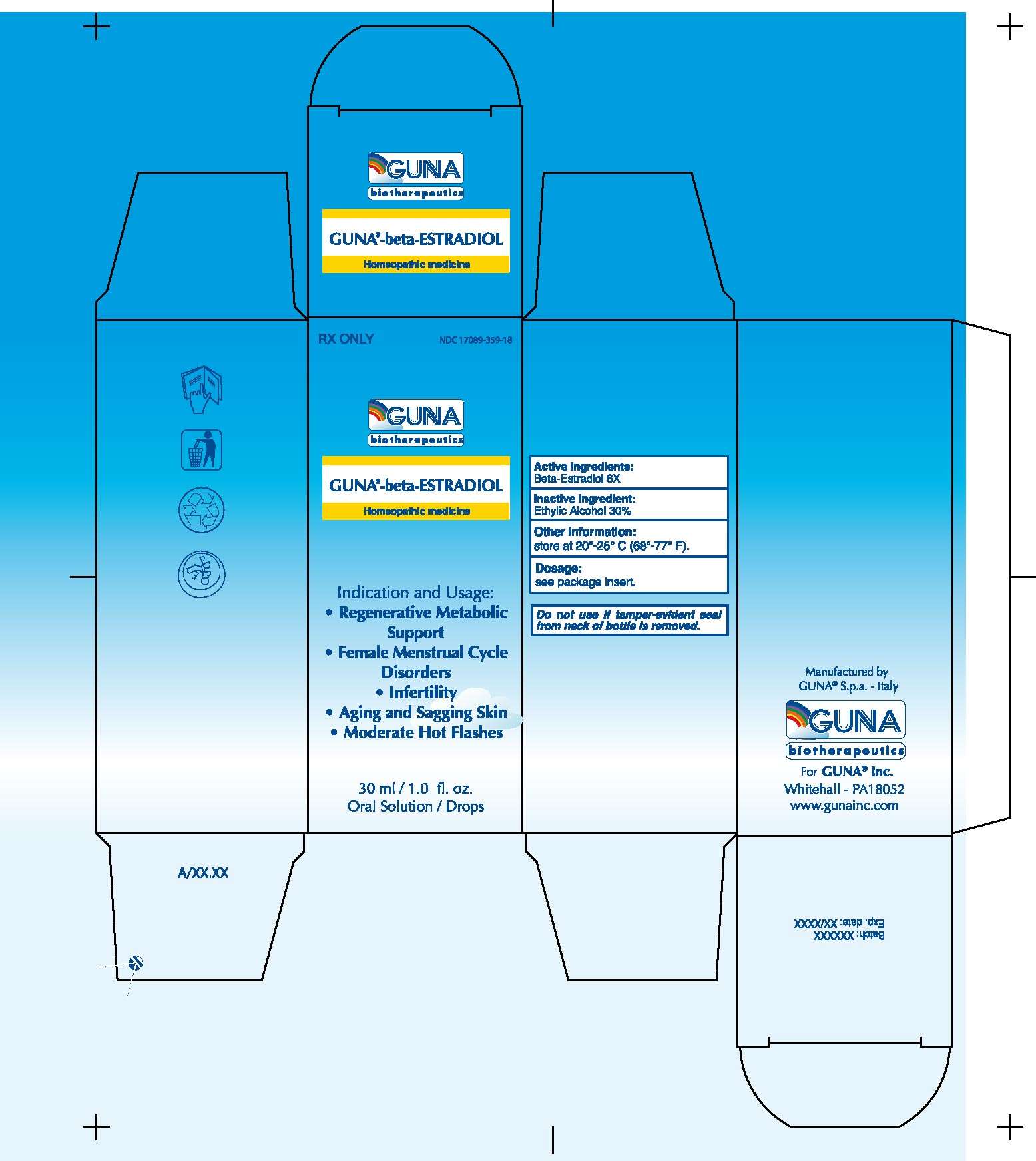
- PACKAGE LABEL
FULL PRESCRIBING INFORMATION
1. INDICATIONS AND USAGE
1.1. Regenerative Metabolic Support (use with GUNA-MATRIX, GUNA LYMPHO and GUNA-CELL)
1.2. Female menstrual cycle disorders (use with GUNA-FEM)
1.3. Infertility (use with GUNA-FEM)
1.4. Aging and sagging skin
1.5. Moderate hot flashes (use with GUNA-FEM)
Administration may vary according to individual needs.
GUNA®-BETA-ESTRADIOL may be used together with other homeopathic medications.
2. DOSAGE AND ADMINISTRATION
3. DOSAGE FORMS AND STRENGTHS
3.1. 30 ml Bottle dropper.
3.2. The ingredient is attenuated according to the procedures stated in the Homeopathic Pharmacopeia of the United States.
Beta-ESTRADIOL 6X.
Inactive Ingredient: Ethylic Alcohol 30%.
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1. Estradiol should not be administered in cases where undiagnosed vaginal bleeding is present; in cases with known, actual or suspected history of breast cancer; during current treatment for metastatic disease; with known or suspected estrogen-dependent neoplasia; with deep vein thrombosis, pulmonary embolism or any history of these conditions; active or recent arterial thromboembolic disease such as stroke or myocardial infarction; or with liver dysfunction or disease.
5.2 Use with caution in patients taking estrogen and/or progesterone therapy.
6. ADVERSE REACTIONS
6.1. None known. (see CONTRAINDICATIONS for hypersensitivity information).
7. DRUG INTERACTIONS
7.1. None Known.
8. USE IN SPECIFIC POPULATIONS
GenderPregnancy
Nursing mothers
Pediatric use
Geriatric use
9. DRUG ABUSE AND DEPENDENCE
9.1. No Known.
10. OVERDOSAGE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
13. NONCLINICAL TOXICOLOGY
14. CLINICAL STUDIES
15. HOW SUPPLIED/STORAGE AND HANDLING
16. PATIENT COUNSELING INFORMATION
16.1. Patients should be informed about Homeopathy and the main differences with conventional clinical approaches.
PACKAGE LABEL
GUNA-Beta-ESTRADIOLESTRADIOL SOLUTION/ DROPS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||