Hannaford Hand Sanitizer
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Hannaford Hand Sanitizer Uses
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- Hannaford Hand Sanitizer Other information
- Inactive Ingredients
- Hannaford bottle label
- Hannaford blister card
- Hannaford shipper label
FULL PRESCRIBING INFORMATION
Active Ingredient
Ethyl alcohol
Purpose
Antiseptic
Hannaford Hand Sanitizer Uses
- For hand sanitizing to decrease bacteria on the skin.
- Recommended for repeated use.
Warnings
- For external use only.
- Do not use in eye area.
When using this product
- Keep out of eyes. In case of eye contact, flush eyes thoroughly with water.
- Avoid contact with broken skin.
Stop use and ask a doctor if
Irritation or redness develops or if condition persists for more than 72 hours.
Keep out of reach of children
If swallowed, get medical help, or contact a Poison control Center immediately.
Directions
- Pump one to three sprays onto palms of hands
- Rub thoroughly over all surfaces of both hands
- Rub hands together briskly until dry
- Children under 6 should be supervised when using this product
- Not recommended for infants
Hannaford Hand Sanitizer Other information
- Store at 200 to 250 C (680 to 770 F)
- Prior to initial use, prime pump by depressing multiple times
- May discolor certain fabrics
- harmful to wood finishes and plastics
Inactive Ingredients
Aloe Vera, D and C Green 5, DI Water, FD and C Yellow 5, Fragrance, Propylene Glycol, Tocopheryl Acetate
Hannaford bottle label
MM9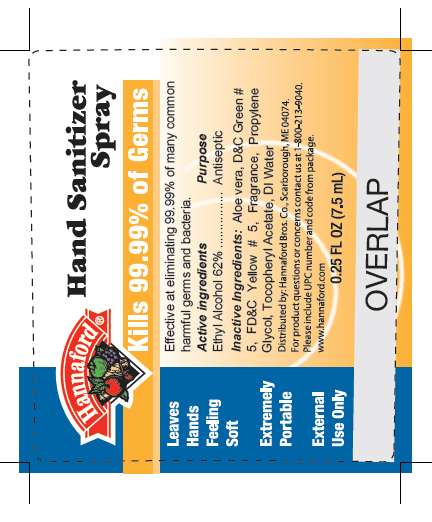
Hannaford blister card
MM10
Hannaford shipper label
MM11
Hannaford Hand SanitizerEthyl alcohol SPRAY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!