Praxair Distribution, Inc.
Helium
FULL PRESCRIBING INFORMATION
UN1046 HELIUM, COMPRESSED USP CONTENTS LOT NUMBER CAUTION! HIGH-PRESSURE GAS. CAN CAUSE RAPID SUFFOCATION. MAY CAUSE DIZZINESS AND DROWSINESS. Store and use with adequate ventilation. Use with equipment rated for cylinder pressure. Use a backflow prevention device in any piping. Cylinder temperature should not exceed 125°F (52°C). Close valve after each use; keep closed even when empty. Always secure cylinder. Install cap, if provided, when not in use per Praxair MSDS form P-4602 and safe practices booklets P-3499 and P-14-153; obtain from your local supplier. FIRST AID: IF INHALED, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, qualified personnel may give oxygen. Call a physician. WARNING! Administration of helium may be hazardous or contraindicated. For use only by or under the supervision of a licensed practitioner who is experienced in the use and administration of helium and is familiar with the indications, effects, dosages, methods and frequency and duration of administration, and with the hazards, contraindications, and side effects and the precautions to be taken. IN CASE OF EMERGENCY: CALL 1-800-645-4633 CAS: 7440-59-7 2 Rx ONLY DISTRIBUTED BY PRAXAIR, INC., DANBURY DO NOT REMOVE THIS LABEL PMG-3 (07/08) Praxair, the flowing airstream design and Medipure are trademarks of Praxair Technology, Inc. DISTRIBUTED BY PRAXAIR, INC, DANBURY, CT 06810-5113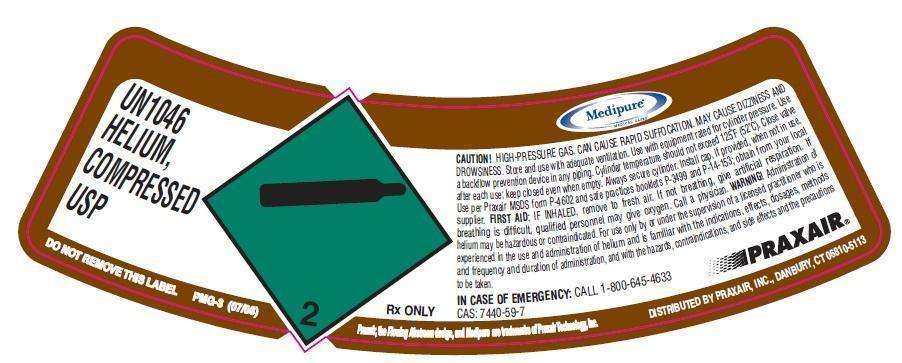
Product Information
|
|
Product Type
|
Human prescription drug label |
Item Code (Source)
|
NDC:59579-005 |
|
Route of Administration
|
RESPIRATORY (INHALATION) |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
Helium Helium |
|
990 mL
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:59579-005-01 |
14696 in 1 CYLINDER |
|
|
|
2 |
NDC:59579-005-02 |
12233 in 1 CYLINDER |
|
|
|
3 |
NDC:59579-005-03 |
9430 in 1 CYLINDER |
|
|
|
4 |
NDC:59579-005-04 |
8240 in 1 CYLINDER |
|
|
|
5 |
NDC:59579-005-05 |
6909 in 1 CYLINDER |
|
|
|
6 |
NDC:59579-005-06 |
6173 in 1 CYLINDER |
|
|
|
7 |
NDC:59579-005-07 |
3823 in 1 CYLINDER |
|
|
|
8 |
NDC:59579-005-08 |
3087 in 1 CYLINDER |
|
|
|
9 |
NDC:59579-005-09 |
2067 in 1 CYLINDER |
|
|
|
10 |
NDC:59579-005-10 |
1048 in 1 CYLINDER |
|
|
|
11 |
NDC:59579-005-11 |
623 in 1 CYLINDER |
|
|
|
12 |
NDC:59579-005-12 |
510 in 1 CYLINDER |
|
|
|
13 |
NDC:59579-005-15 |
368 in 1 CYLINDER |
|
|
|
14 |
NDC:59579-005-16 |
113 in 1 CYLINDER |
|
|
|
15 |
NDC:59579-005-17 |
170 in 1 CYLINDER |
|
|
|
16 |
NDC:59579-005-18 |
6768 in 1 CYLINDER |
|
|
|
17 |
NDC:59579-005-19 |
2209 in 1 CYLINDER |
|
|
|
18 |
NDC:59579-005-20 |
821 in 1 CYLINDER |
|
|
|
19 |
NDC:59579-005-13 |
538 in 1 CYLINDER |
|
|
|
20 |
NDC:59579-005-14 |
142 in 1 CYLINDER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
NDA |
NDA205912 |
2007-12-01 |
|
|
