HemeNatal OB plus DHA
HemeNatal OB + DHA (Prenatal Multivitamin - Multimineral Tablets and Omega-3 Fatty Acid Softgel Capsules)
FULL PRESCRIBING INFORMATION: CONTENTS*
- HEMENATAL OB PLUS DHA DESCRIPTION:
- HEMENATAL OB PLUS DHA INDICATIONS AND USAGE:
- HEMENATAL OB PLUS DHA CONTRAINDICATIONS:
- WARNINGS AND PRECAUTIONS:
- HEMENATAL OB PLUS DHA ADVERSE REACTIONS:
- HEMENATAL OB PLUS DHA DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
FULL PRESCRIBING INFORMATION
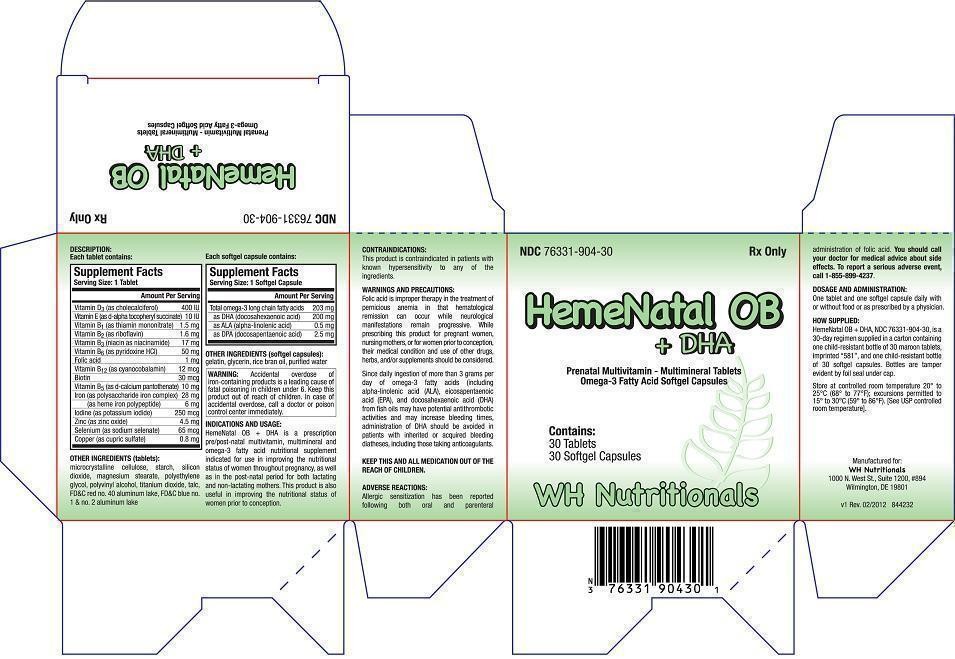
DESCRIPTION:
Each tablet contains:|
Supplement Facts
Serving Size: 1 Tablet Amount Per Serving Vitamin D3 (as cholecalciferol) Vitamin E (as d-alpha tocopheryl succinate) Vitamin B1 (as thiamin mononitrate) Vitamin B2 (as riboflavin) Vitamin B3 (niacin as niacinamide) Vitamin B6 (as pyridoxine HCl) Folic acid Vitamin B12 (as cyanocobalamin) Biotin Vitamin B5 (as d-calcium pantothenate) Iron (as polysaccharide iron complex) (as heme iron polypeptide) Iodine (as potassium iodide) Zinc (as zinc oxide) Selenium (as sodium selenate) Copper (as cupric sulfate) |
400 IU 10 IU 1.5 mg 1.6 mg 17 mg 50 mg 1 mg 12 mcg 30 mcg 10 mg 28 mg 6 mg 250 mcg 4.5 mg 65 mcg 0.8 mg |
|
Supplement Facts Serving Size: 1 Softgel Capsule Amount Per Serving Total omega-3 long chain fatty acids as DHA (docosahexaenoic acid) as ALA (alpha-linolenic acid) as DPA (docosapentaenoic acid) |
203 mg 200 mg 0.5 mg 2.5 mg |
OTHER INGREDIENTS (softgel capsules):
INDICATIONS AND USAGE:
CONTRAINDICATIONS:
WARNINGS AND PRECAUTIONS:
|
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. |
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
ADVERSE REACTIONS:
You should call your doctor for medical advice about side effects. To report a serious adverse event, call 1-855-899-4237.DOSAGE AND ADMINISTRATION:
HOW SUPPLIED:
HemeNatal OB plus DHACHOLECALCIFEROL, .ALPHA.-TOCOPHEROL SUCCINATE, D-, THIAMINE MONONITRATE, RIBOFLAVIN, NIACINAMIDE, PYRIDOXINE HYDROCHLORIDE, FOLIC ACID, CYANOCOBALAMIN, BIOTIN, CALCIUM PANTOTHENATE, IRON SUCROSE, HEME IRON POLYPEPTIDE, POTASSIUM IODIDE, ZINC OXIDE, SODIUM SELENATE, CUPRIC SULFATE, DOCONEXENT, LINOLENIC ACID, OSBOND ACID KIT
| ||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!