Hemorrodil Unguento Plus
Hemorrodil Unguento Plus
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Hemorrodil Unguento Plus Uses
- Warnings
- Instructions
- Inactive Ingredients
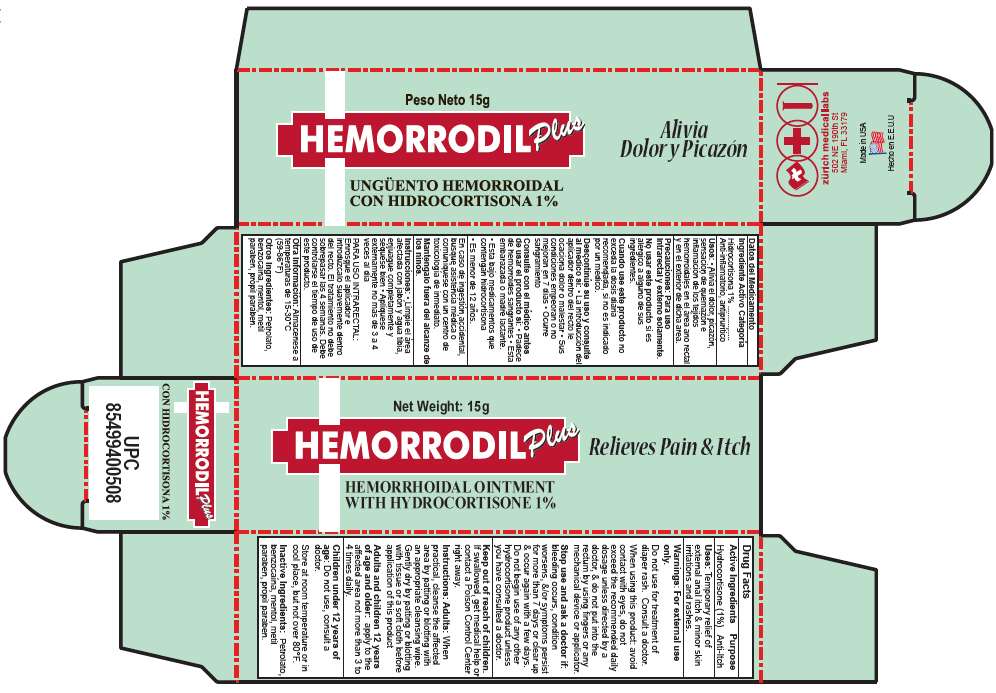
- PRINCIPAL DISPLAY PANEL - 15 g Tube Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredients
Hydrocortisone (1%)
Purpose
Anti-Itch
Hemorrodil Unguento Plus Uses
Temporary relief of external anal itch & minor skin irritations and rashes.
Warnings
For external use only.
Do not use for treatment of diaper rash. Consult a doctor.
When using this product: avoid contact with eyes, do not exceed the recommended daily dosage unless directed by a doctor, & do not put into the rectum by using fingers or any mechanical device or applicator.
Stop use and ask a doctor if: bleeding occurs, condition worsens, &/or symptoms persist for more than 7 days or clear up & occur again with a few days. Do not begin use of any other hydrocortisone product unless you have consulted a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Instructions
Adults
When practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with tissue or a soft cloth before application of this product
Adults and children 12 years of age and older: apply to the affected area not more than 3 to 4 times daily.
Children under 12 years of age
Do not use, consult a doctor.
Store at room temperature or in cool place, but not over 80°F.
Inactive Ingredients
Petrolato, benzocaína, mentol, metil paraben, propil paraben.
PRINCIPAL DISPLAY PANEL - 15 g Tube Carton
Net Weight: 15g
HEMORRODIL Plus
Relieves Pain & Itch
HEMORRHOIDAL OINTMENT
WITH HYDROCORTISONE 1%
Hemorrodil Unguento PlusHydrocortisone OINTMENT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||