HepaGam B
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use HepaGam B safely and effectively. See full prescribing information for HepaGam B. HepaGam B[Hepatitis B Immune Globulin Intravenous (Human)],Sterile Solution for Intravenous or Intramuscular Injection Solvent/Detergent Treated and Filtered. >312 IU/mL (Measured potency stamped on the vial label)Initial U.S. Approval: 2006RECENT MAJOR CHANGESIndications and Usage, Prevention of Hepatitis B recurrence following Liver Transplantation (1.1) 4/2007Dosage and Administration, Prevention of Hepatitis B recurrence following Liver Transplantation (2.1) 4/2007INDICATIONS AND USAGE Prevention of Hepatitis B recurrence following Liver Transplantation in HBsAg-positive liver transplant patients (1.1). Postexposure Prophylaxis in the following settings: Acute Exposure to Blood Containing HBsAg Perinatal Exposure of Infants Born to HBsAg-positive Mothers Sexual Exposure to HBsAg-positive Persons Household Exposure to Persons with Acute HBV Infection (1.2) DOSAGE AND ADMINISTRATION Prevention of Hepatitis B recurrence following liver transplantation (2.1) HepaGam B is administered intravenously at doses of 20,000 IU (calculated from the measured potency stamped on the vial label) according to the following regimen to attain serum anti-HBs > 500 IU/L: Anhepatic Phase Week 1 Post-Operative Weeks 2-12 Post-Operative Month 4 onwards First dose Daily from Day 1-7 Every two weeks from Day 14 Monthly Regularly monitor serum anti-HBs to allow for treatment adjustments Postexposure Prophylaxis (2.2) HepaGam B must be administered intramuscularly only as directed below: Acute Exposure to Blood Containing HBsAg 0.06 mL/kg Administer as soon as possible after exposure and within 24 hours if possible Perinatal Exposure of Infants Born to HBsAg-positive Mothers 0.5mL Administer after physiologic stabilization of the infant and preferably within 12 hours of birth. Sexual Exposure to HBsAg-positive Persons 0.06 mL/kg Administer HepaGam B within 14 days of the last sexual contact or if sexual contact with the infected person will continue Household Exposure to Persons with Acute HBV Infection 0.5mL Infants < 12 months: Administer HepaGam B + Hepatitis B vaccine if primary caregiver has acute HBV infection. DOSAGE FORMS AND STRENGTHS1.0 mL sterile solution; single use vial (> 312 IU/mL) in a carton. (3)5.0 mL sterile solution; single use vial (> 312 IU/mL) in a carton. (3)The measured potency of each lot is stamped on the vial label CONTRAINDICATIONS History of anaphylactic or severe systemic reactions to human globulins (4) IgA deficient individuals may have the potential to develop IgA antibodies and have an anaphylactoid reaction. (4) IM injections may be contraindicated in patients with coagulation disorders (4) WARNINGS AND PRECAUTIONS Risk of transmission of infectious agents from human plasma (5.1) Anaphylactic Precautions (5.2) Interference with non-glucose-specific blood glucose monitoring systems may result in elevated glucose readings that can lead to untreated hypoglycemia or inappropriate insulin administration (5.3) Infusion rate precautions (5.5) IM injections may be contraindicated in patients with coagulation disorders (5.6) Side EffectsThe most common expected adverse drug reactions for intravenous immune globulins like HepaGam B are chills, fever, headaches, vomiting, allergic reactions, nausea, arthralgia and moderate low back pain. (6) To report SUSPECTED ADVERSE REACTIONS, contact Cangene Corporation at 1-800-768-2304 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS Efficacy of live attenuated virus vaccines may be impaired by immune globulin administration; revaccination may be necessary. (7.1) Antibodies in HepaGam B may interfere with some serological tests (7.2) Maltose in HepaGam B may interfere with non-glucose specific blood glucose testing systems. (7.3) USE IN SPECIFIC POPULATIONS Pregnant women: Only if indicated (8.1) Nursing mothers: Caution should be exercised (8.2)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 HEPAGAM B INDICATIONS AND USAGE
- 2 HEPAGAM B DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 HEPAGAM B CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 HEPAGAM B ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 HEPAGAM B DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- Principal Display Panel
- Principal Display Panel
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Prevention of Hepatitis B recurrence following Liver Transplantation
HepaGam B®, Hepatitis B Immune Globulin Intravenous (Human), is indicated for the prevention of hepatitis B recurrence following liver transplantation, in HbsAg-positive liver transplant patients.
HepaGam B should be administered intravenously for this indication.
1.2 Postexposure Prophylaxis
HepaGam B is indicated for the treatment of acute exposure to blood containing HbsAg, perinatal exposure of infants born to HbsAg-positive mothers, sexual exposure to HbsAgpositive
persons and household exposure to persons with acute HBV infection in the following settings:
Following either parenteral exposure (needlestick, bite, sharps), direct mucous membrane contact (accidental splash), or oral ingestion (pipetting accident), involving HbsAg-positive materials such as blood, plasma or serum1,2.
Perinatal Exposure of Infants Born to HbsAg-positive Mothers
Infants born to mothers positive for HbsAg with or without HbeAg1.
Sexual Exposure to HbsAg-positive Persons
Sexual partners of HbsAg-positive persons1,2.
Household Exposure to Persons with Acute HBV Infection
Infants less than 12 months old whose mother or primary caregiver is positive for HbsAg. Other household contacts with an identifiable blood exposure to the index patient.
HepaGam B is indicated for intramuscular use only for these post-exposure prophylaxis indications.
2 DOSAGE AND ADMINISTRATION
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration; if these are seen, vials should not be used. During preparation, do not shake vials; avoid foaming.
Any vial of HepaGam B that has been entered should be used promptly. Do not reuse or save for future use. This product contains no preservative; therefore, partially used vials should be discarded immediately.
2.1 Prevention of Hepatitis B recurrence following liver transplantation
For the prevention of hepatitis B recurrence following liver transplantation in HBsAg positive liver transplant patients, HepaGam B is administered intravenously according to a set dosing regimen designed to attain serum levels of antibodies to hepatitis B surface antigen (anti-HBs) greater than 500 IU/L3.
Based upon the HepaGam B clinical trial, patients should receive 20,000 IU/dose [see Clinical Trials in Liver Transplant Patients (14.1) ]. The volume of each 20,000 IU dose should be calculated from the measured potency of the particular lot of HepaGam B as stamped on the vial label.
The first dose should be administered concurrently with the grafting of the transplanted liver (the anhepatic phase) with subsequent dosing as recommended in Table 1.
|
Anhepatic Phase |
Week 1 Post-Operative |
Weeks 2-12 Post-Operative |
Month 4 onwards |
| First dose | Daily from Day 1-7 | Every two weeks from Day 14 | Monthly |
* Each dose should contain 20,000 IU calculated from the measured potency as stamped on the vial label [see Dosage Forms and Strengths (3) ].
HepaGam B dose adjustments may be required in patients who fail to reach anti-HBs levels of 500 IU/L within the first week post-liver transplantation4. Patients who have surgical bleeding or abdominal fluid drainage (> 500 mL) or patients who undergo plasmapheresis are particularly susceptible to extensive loss of circulated anti-HBs. In these cases, the dosing regimen should be increased to a half-dose (10,000 IU calculated from the measured potency as stamped on the vial label) intravenously every 6 hours until the target anti-HBs is reached.
Hepatitis B Immune Globulin (HBIG) products are most effective in patients with no or low levels of HBV replication at the time of transplantation5.
Regular monitoring of serum HBsAg and levels of anti-HBs antibody should be performed pre-infusion to track treatment response and allow for treatment adjustment.
HepaGam B should be prepared for intravenous administration under aseptic conditions. HepaGam B should be administered through a separate intravenous line using an intravenous administration set via infusion pump.
The rate of administration should be set at 2 mL per minute.
The rate of infusion should be decreased to 1 mL per minute or slower if the patient develops discomfort, infusion-related adverse events or there is concern about the speed of infusion.
2.2 Postexposure Prophlyaxis
For postexposure prophylaxis indications, HepaGam B must be administered intramuscularly only as directed below.
It is important to use a separate vial, sterile syringe, and needle for each individual patient, to prevent transmission of infectious agents from one person to another.
HepaGam B may be administered at the same time (but at a different site), or up to one month preceding hepatitis B vaccination without impairing the active immune response to Hepatitis B Vaccine1,2.
Acute Exposure to Blood Containing HBsAg
Table 2 summarizes prophylaxis for percutaneous (needlestick, bite, sharps), ocular, or mucous membrane exposure to blood according to the source of exposure and vaccination status of the exposed person. For greatest effectiveness, passive prophylaxis with HepaGam B should be given as soon as possible after exposure, as its value after seven days following exposure is unclear1,2. An injection of 0.06 mL/kg of body weight should be administered intramuscularly as soon as possible after exposure, and within 24 hours if possible. Consult the Hepatitis B Vaccine package insert for dosage information regarding the vaccine.
For persons who refuse Hepatitis B Vaccine or are known non-responders to vaccine, a second dose of HepaGam B should be given one month after the first dose2.
| Source | Exposed Person | |
| Unvaccinated | Vaccinated | |
| HBsAg-positive | 1. Hepatitis B Immune Globulin Intravenous (Human) (HBIGIV) x 1 immediately 2. Initiate HB vaccine series  |
1. Test exposed person for anti-HBs 2. If inadequate antibody    |
| Known Source – High Risk for HBsAg-positive | 1. Initiate HB vaccine series 2. Test source of HBsAg. If positive, Hepatitis B immune Globulin Intravenous (Human) (HBIGIV) x 1 |
1. Test source for HBsAg only if exposed is vaccine nonresponder; if source is HBsAg-positive, give Hepatitis B Immune Globulin Intravenous (Human) x 1 immediately plus either HB vaccine booster dose, or a second dose of HBIGIV  |
| Known Source – Low Risk for HBsAg-positive | Initiate HB vaccine series | Nothing required |
| Unknown Source | Initiate HB vaccine series | Nothing required |
Prophylaxis of Infants Born to Mothers who are Positive for HBsAg with or without HBeAg
Table 3 contains the recommended schedule of Hepatitis B prophylaxis for infants born to mothers that are either known to be positive for HBsAg or have not been screened. Infants born to mothers known to be HBsAg-positive should receive 0.5 mL HepaGam B after physiologic stabilization of the infant and preferably within 12 hours of birth. The Hepatitis B Vaccine series should be initiated simultaneously, if not contraindicated, with the first dose of the vaccine given concurrently with the HepaGam B, but at a different site. Subsequent doses of the vaccine should be administered in accordance with the recommendations of the manufacturer. Women admitted for delivery, who were not screened for HBsAg during the prenatal period, should be tested. While test results are pending, the newborn infant should receive Hepatitis B Vaccine within 12 hours of birth (see manufacturers’ recommendations for dose). If the mother is later found to be HBsAg-positive, the infant should receive 0.5 mL HepaGam B as soon as possible and within seven days of birth; however, the efficacy of HepaGam B administered after 48 hours of age is not known6. Testing for HBsAg and anti-HBs is recommended at 12-15 months of age. If HBsAg is not detectable and anti-HBs is present, the child has been protected1.
| Age of Infant | ||
| Administer | Infant born to mother known to be HBsAg-positive | Infant born to mother not screened for HBsAg |
First Vaccination Hepatitis B Immune Globulin Intravenous (Human)  |
Birth (within 12 hours) Birth (within 12 hours) |
Birth (within 12 hours) If mother is found to be HBsAg-positive, administer dose to infant as soon as possible, not later than 1 week after birth |
Second Vaccination |
1 month | 1-2 months |
Third Vaccination |
6 months |
6 months |
Sexual Exposure to HBsAg-positive Persons
All susceptible persons whose sexual partners have acute hepatitis B infection should receive a single dose of HepaGam B (0.06 mL/kg) and should begin the Hepatitis B Vaccine series, if not contraindicated, within 14 days of the last sexual contact or if sexual contact with the infected person will continue. Administering the vaccine with HepaGam B may improve the efficacy of post exposure treatment. The vaccine has the added advantage of conferring long-lasting protection1,2.
Household Exposure to Persons with Acute HBV Infection
Prophylaxis of an infant less than 12 months of age with 0.5 mL HepaGam B and Hepatitis B Vaccine is indicated if the mother or primary caregiver has acute HBV infection. Prophylaxis of other household contacts of persons with acute HBV infection is not indicated unless they had an identifiable blood exposure to the index patient, such as by sharing toothbrushes or razors. Such exposures should be treated like sexual exposures. If the index patient becomes an HBV carrier, all household contacts should receive Hepatitis B Vaccine1,2.
3 DOSAGE FORMS AND STRENGTHS
HepaGam B, Hepatitis B Immune Globulin Intravenous (Human), is a sterile solution of purified gamma globulin (5% or 50 mg/mL) containing anti-HBs. Each vial contains greater than 312 IU/mL of anti-HBs and is supplied in a carton containing a 1.0 mL single use vial or a carton containing a 5.0 mL single use vial. The measured potency of each lot is stamped on the vial label.
To ensure that the label claim of >312 IU/mL is maintained over the product shelf life, a higher potency of 550 IU/mL is targeted at the time of manufacture. As with other specific immune globulin products, this higher target potency is a manufacturing requirement to account for variability in the potency assay and changes in potency over time. The potency assay has a relative standard deviation (RSD) of approximately 10%. Based on statistical assessment of manufactured lots with a target potency of 550 IU/mL, the actual potency test result may vary from approximately 400 to 700 IU/mL (3x RSD). The measured potency is provided on the container label. Due to the inherent variability of the potency assay, dosing for the prevention of hepatitis B recurrence following liver transplantation should be calculated from the measured potency of the particular lot of HepaGam B as stamped on the vial label [see Dosage and Administration (2.1) ].
4 CONTRAINDICATIONS
Individuals known to have anaphylactic or severe systematic reactions associated with the parenteral administration of human globulin preparations should not receive HepaGam B, Hepatitis B Immune Globulin Intravenous (Human), or any other human immune globulin. HepaGam B contains less than 40 micrograms/mL of IgA. Individuals who are deficient in IgA may have the potential to develop IgA antibodies and have an anaphylactoid reaction. The physician must weigh the potential benefit of treatment with HepaGam B against the potential for hypersensitivity reactions.
For postexposure prophylaxis indications, HepaGam B must be administered intramuscularly only. In patients who have severe thrombocytopenia or any coagulation disorder that would contraindicate intramuscular injections, HepaGam B should be given only if the expected benefits outweigh the potential risks.
5 WARNINGS AND PRECAUTIONS
5.1 General
HepaGam B is made from human plasma. Products made from human plasma may contain infectious agents, such as viruses and theoretically, the Creutzfeldt-Jakob disease agent. The risk that such products can transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and/or removing certain viruses. The HepaGam B manufacturing process includes a solvent/detergent treatment step (using tri-n-butyl phosphate and Triton X-100) that is effective in inactivating known enveloped viruses such as HBV, HCV, and HIV. HepaGam B is filtered using a Planova 20N Virus Filter that is effective in reducing the levels of some enveloped and non-enveloped viruses. These two processes are designed to increase product safety. Despite these measures, such products can still potentially transmit disease. There is also the possibility that unknown infectious agents may be present in such products. ALL infections thought by a physician to have been transmitted by this product should be reported by the physician or other healthcare provider to Cangene Corporation at 1-800-768-2304. The physician should discuss the risks and benefits of this product with the patient.
5.2 Anaphylactic Precautions
Although allergic reactions have not been reported following HepaGam B administration [see Adverse Reactions (6.2) ], the product should be administered only in a setting where appropriate equipment and personnel trained in the management of acute anaphylaxis are available. If hypotension or anaphylaxis occurs, the administration of HepaGam B should be discontinued immediately and supportive care given as needed.
5.3 Interference with Blood Glucose Testing
The maltose contained in HepaGam B can interfere with some types of blood glucose monitoring systems, i.e., those based on the glucose dehydrogenase pyrroloquinequinone (GDH-PQQ) method. This can result in falsely elevated glucose readings and, consequently, in the inappropriate administration of insulin, resulting in life-threatening hypoglycemia. Cases of true hypoglycemia may go untreated if the hypoglycemic state is masked by falsely elevated results.
5.4 Monitoring: Serum anti-HBs Antibody Levels
Liver transplant patients should be monitored regularly for serum anti-HBs antibody levels using a quantitative assay [see Dosage and Administration (2.1) ].
5.5 Infusion Reactions
Certain adverse drug reactions may be related to the rate of infusion. The recommended infusion rate given under Dosage and Administration (2.1) must be closely followed. Patients must be closely monitored and carefully observed for any symptoms throughout the infusion period and immediately following an infusion.
5.6 Coagulation Disorders
For postexposure prophylaxis indications, HepaGam B must be administered intramuscularly only. In patients who have severe thrombocytopenia or any coagulation disorder that would contraindicate intramuscular injections, HepaGam B should be given only if the expected benefits outweigh the potential risks.
6 ADVERSE REACTIONS
6.1 Overall Adverse Reaction Profile
The most common expected adverse drug reactions for intravenous immune globulins like HepaGam B are chills, fever, headaches, vomiting, allergic reactions, nausea, arthralgia and moderate low back pain7,8. In a clinical trial in liver transplant patients, 2 adverse drug reactions of tremor and hypotension were reported in 2 of 14 patients who received intravenous infusions of HepaGam B8. In studies with healthy volunteers, only 1 adverse drug reaction of nausea was reported in the 70 adult subjects who received an intramuscular administration of HepaGam B8.
Although no anaphylactic reactions have been reported following HepaGam B administration, anaphylactic reactions have been reported following the administration of other immune globulin products on rare occasions [see Warnings and Precautions (5.2) ].
6.2 Side Effects in Clinical Trials
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Hepatitis B-Related Liver Transplantation
In an ongoing clinical trial, only 2 adverse drug reactions occurred following the 313 (<1%) HepaGam B infusions in 14 liver transplant patients. A listing of all adverse events (including those assessed as unrelated to study drug) occurring in >10% of patients are summarized in Table 4 below. These adverse events were reported in an interim analysis from a one-year Phase 3 clinical trial examining HepaGam B for the prevention of hepatitis B recurrence following liver transplantation. This study utilized the recommended dosing regimen outlined in Table 1 [see Dosage and Administration (2.1) ]. The 2 attributed adverse drug reactions of tremor and hypotension were reported in 2 patients. All reactions were associated with a single HepaGam B infusion during the first week post-transplant. All reactions resolved on the same day and did not recur with subsequent HepaGam B infusions.
| Adverse Event by system organ class | Number of AEs (in number of patients) N=14 |
| Blood and lymphatic systems disorder - Splenomegaly |
8 (6) |
| Eye disorders - Presbyopia |
2 (2) |
| Gastrointestinal disorders - Aphthous stomatitis - Diarrhoea - Dyspepsia - Gingival Hyperplasia |
3 (3) 10 (8) 5 (5) 3 (3) |
| General disorders - Fatigue - Oedema peripheral - Pyrexia |
6 (6) 3 (2) 3 (3) |
| Hepatobiliary disorders - Hepatobiliary disease |
3 (3) |
| Immune system disorders - Liver transplant rejection |
7 (5) |
| Infections and infestations - Diarrhoea Infectious - Pneumonia - Sepsis |
2 (2) 2 (2) 3 (2) |
| Metabolism and nutrition disorders - Hyperglycaemia |
4 (4) |
| Musculoskeletal - Back pain |
2 (2) |
| Nervous system disorders - Amnesia - Essential Tremor - Headache |
2 (2) 6 (2) 15 (9) |
| Psychiatric disorders - Agitation |
2 (2) |
| Renal and urinary disorders - Nocturia |
2 (2) |
| Respiratory, thoracic and mediastinal - Pleural effusion |
3 (3) |
| Skin and subcutaneous tissue disorders - Pruritus - Rash |
3 (3) 2 (2) |
| Vascular disorders - Hypertension - Hypotension |
8 (7) 2 (2) |
Healthy Volunteer Studies
Seventy healthy male and female volunteers received a single dose of HepaGam B intramuscularly in clinical trials8. Seventeen (17) subjects reported 30 adverse events following administration of HepaGam B. The most frequently reported adverse events included 4 subjects (6%) who experienced headache, 7 subjects (10%) who had cold symptoms or flu and 2 subjects (3%) who experienced lightheadedness/fainted. The majority of events were reported as mild and were not related to study drug. One adverse event, an episode of nausea, was considered to be drug related. There were no serious adverse events reported. A similar number of subjects in the comparator groups reported adverse events.
6.3 Postmarketing Experience
As of April 2007, there have been no postmarketing adverse events reported for HepaGam B administered IM
7 DRUG INTERACTIONS
7.1 Live Attenuated Virus Vaccines
Immune globulin administration may impair the efficacy of live attenuated virus vaccines such as measles, rubella, mumps and varicella1,2,7. Vaccination with live virus vaccines should be deferred until approximately three months after administration of HepaGam B, Hepatitis B Immune Globulin Intravenous (Human). Persons who received HepaGam B less than 14 days after live virus vaccination should be revaccinated 3 months after the administration of the immune globulin, unless serologic test results indicate that antibodies were produced1,2.
There are no available data on drug interactions of HepaGam B with other medications.
7.2 Drug-Laboratory Interactions: Serological Testing
Antibodies present in HepaGam B may interfere with some serological tests. After administration of immune globulins like HepaGam B, a transitory increase of passively transferred antibodies in the patient’s blood may result in misleading positive results in serological testing (e.g. Coombs' test).
7.3 Drug-Laboratory Interactions: Blood Glucose Testing
HepaGam B contains maltose which can interfere with certain types of blood glucose monitoring systems. [See Warnings and Precautions (5.3).] Only testing systems that are glucose-specific should be used in patients receiving HepaGam B. This interference can result in falsely elevated glucose readings that can lead to untreated hypoglycemia or to inappropriate insulin administration, resulting in life-threatening hypoglycemia.
The product information of the blood glucose testing system, including that of the test strips, should be carefully reviewed to determine if the system is appropriate for use with maltose-containing parenteral products. If any uncertainty exists, contact the manufacturer of the testing system to determine if the system is appropriate for use with maltose-containing parenteral products.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy Category C
Animal reproduction studies have not been conducted with HepaGam B. It is also not known whether HepaGam B can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. HepaGam B should be given to a pregnant woman only if clearly indicated.
8.2 Nursing Mothers
It is not known whether HepaGam B is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when HepaGam B is administered to a nursing mother.
10 OVERDOSAGE
Consequences of an overdose are not known. Although no data are available, clinical experience reported with other intravenously administered human immune globulin preparations suggests that doses above 1 g immune globulin/kg body weight are tolerated. For intramuscular administration of HepaGam B, the only manifestations of overdose would be pain and tenderness at the injection site.
11 DESCRIPTION
HepaGam B, Hepatitis B Immune Globulin Intravenous (Human), is a solvent/detergent-treated sterile solution of purified gamma globulin containing anti-HBs. It is prepared from plasma donated by healthy, screened donors with high titers of anti-HBs that is purified by an anion-exchange column chromatography manufacturing method9,10. HepaGam B is formulated as a 5% (50 mg/mL) protein solution with 10% maltose and 0.03% polysorbate 80 at pH 5.6. It is available in 1 mL and 5 mL single dose vials. The product appears as a clear to opalescent liquid. It contains no preservatives and is intended for single use. HepaGam B may be administered intravenously or intramuscularly dependent upon indication [see Indications and Usage (1.) ]. The source plasma used in the manufacture of this product was tested by FDA licensed Nucleic Acid testing (NAT) for HIV-1 and HCV and found to be negative. An investigational NAT for HBV was performed on all Source Plasma used, and found to be negative; however, the significance of a negative result has not been established. Plasma also has been tested by inprocess NAT for hepatitis A virus (HAV) and parvovirus B19 (B19) via minipool testing and the limit for B19 in the manufacturing pool is set not to exceed 104 IU of B19 DNA per mL.
The manufacturing process contains two steps implemented specifically for virus clearance. The solvent and detergent step (using tri-n-butyl phosphate and Triton X-100) is effective in the inactivation of enveloped viruses, such as hepatitis B, hepatitis C and HIV11. Virus filtration, using a Planova 20N virus filter, is effective for the removal of viruses based on their size, including some non-enveloped viruses12. These two viral clearance steps are designed to increase product safety by reducing the risk of transmission of enveloped and non-enveloped viruses. In addition to these two specific steps, the process step of anion-exchange chromatography was identified as contributing to the overall viral clearance capacity for small non-enveloped viruses.
The inactivation and reduction of known enveloped and non–enveloped model viruses were validated in laboratory studies as summarized in Table 5. The viruses employed for spiking studies were selected to represent those viruses that are potential contaminants in the product, and to represent a wide range of physiochemical properties in order to challenge the manufacturing process’s ability for viral clearance in general.
| Enveloped | Enveloped | Non-Enveloped | |||||
| Genome | RNA | DNA | RNA | DNA | |||
| Virus | HIV-1 | BVDV | PRV | HAV | EMC | MMV | PPV |
| Family | retro | flavi | herpes | picorna | parvo | ||
| Size (nm) | 80-100 | 50-70 | 120-200 | 25-30 | 30 | 20-25 | 18-24 |
| Anion Exchange Chromatography(partitioning) | Not evaluated | 2.3 | n.e. | 3.4 | n.e. | ||
| 20N Filtration(size exclusion) | ≥ 4.7 | ≥ 3.5 | ≥ 5.6  |
n.e. | 4.8 | n.e. | 4.1 |
| Solvent/Detergent (inactivation) | ≥ 4.7 | ≥ 7.3 | ≥ 5.5 | Not evaluated | |||
| Total Reduction (log10) | ≥ 9.4 | ≥ 10.8 | ≥ 11.1 | 2.3 | 4.8 | 3.4 | 4.1 |
Abbreviations:
HIV-1: human immunodeficiency virus-1; relevant virus for human immunodeficiency virus-1 and model for HIV-2
BVDV: bovine viral diarrhea virus; model virus for hepatitis C virus (HCV) and West Nile virus (WNV)
PRV: pseudorabies virus; model for large enveloped DNA viruses, including herpes
HAV: human hepatitis A virus; relevant virus for HAV and model for small non-enveloped viruses in general
EMC: encephalomyocarditis virus; model for HAV and for small non-enveloped viruses in general
MMV: murine minute virus; model for human parvovirus B19 and for small non-enveloped viruses in general
PPV: porcine parvovirus; model for human parvovirus B19 and for small non-enveloped viruses in general
n.e.: not evaluated
a The PRV was retained by the 0.1 μm pre-filter during the virus validation. Since manufacturing employs a 0.1 μm pre-filter before the 20N filter, the claim of ≥ 5.6 reduction is considered applicable.
The product potency is expressed in international units (IU) by comparison to the World Health Organization (WHO) standard Hepatitis B Immune Globulin. Each vial contains greater than 312 IU/mL. The measured potency of each lot is stamped on the vial label [see Dosage Forms and Strengths (3.) ].
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
HBIGIV products provide passive immunization for individuals exposed to the hepatitis B virus, by binding to the surface antigen and reducing the rate of hepatitis B infection13-16.
Hepatitis B virus reinfection following liver transplantation is the consequence of exposure of the new liver graft to hepatitis B virus. Reinfection may occur immediately at the time of liver reperfusion due to circulating virus or later from virus retained in extrahepatic sites.
The mechanism whereby hepatitis B Immune globulin (HBIG) protects the transplanted liver against HBV reinfection is not well understood. HBIG may protect naive hepatocytes against infection through blockage of a putative HBV receptor17. Alternatively, HBIG may neutralize circulating virions through immune precipitation and immune complex formation or may trigger an antibody-dependent cell-mediated cytotoxicity response resulting in target cell lysis17, 18. In addition, HBIG has been reported to bind to hepatocytes and interact with HBsAg within cells19. Regardless of the mechanism, there is evidence of a dose-dependent response to HBIG treatment5,8.
12.2 Postexposure Prophylaxis
Clinical studies conducted prior to 1983 with hepatitis B immune globulins similar to HepaGam B demonstrated the advantage of simultaneous administration of hepatitis B vaccine and Hepatitis B Immune Globulin (Human), by the i.m. route. The Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) advises that the combination prophylaxis be provided following certain instances of hepatitis B exposure.1,2 Recommendations on post-exposure prophylaxis are based on available efficacy data, primarily from studies in neonates1,2. Cases of hepatitis B are rarely seen following exposure to HBV in persons with pre-existing anti-HBs antibodies.
Infants born to HBsAg-positive mothers are at risk of being infected with HBV and becoming chronic carriers1. The risk is especially great if the mother is also HBeAg-positive1. For an infant with perinatal exposure to an HBsAg-positive and HBeAg-positive mother, a regimen combining one dose of Hepatitis B Immune Globulin (Human) at birth with the hepatitis B vaccine series started soon after birth has been shown to be 85-98% effective in preventing development of the HBV carrier state1,2. Regimens involving either multiple doses of Hepatitis B Immune Globulin (Human) alone or the vaccine series alone have a 70-75% efficacy, while a single dose of Hepatitis B Immune Globulin (Human) alone has 50% efficacy1,2.
Since infants have close contact with primary caregivers and have a higher risk of becoming HBV carriers after acute HBV infection, prophylaxis of an infant less than 12 months of age with Hepatitis B Immune Globulin (Human) and Hepatitis B Vaccine is indicated if the mother or primary caregiver has acute HBV infection1.
Sexual partners of HBsAg-positive persons are at increased risk of acquiring HBV infection. A single dose of Hepatitis B immune Globulin (Human) is 75% effective if administered within two weeks of the last sexual exposure to a person with acute hepatitis B1,2.
HBV infection is a well-recognized risk to health-care personnel (HCP). The risk of HBV infection is primarily related to the degree of contact with blood in the work place and also to the hepatitis B e antigen (HBeAg) status of the source person. In studies of HCP who sustained injuries from needles contaminated with blood containing HBV, the risk of developing clinical hepatitis if the blood was hepatitis B surface antigen (HBsAg) and HBeAg-positive was 22%-31%; the risk of developing serologic evidence of HBV infection was 37%-62%. In comparison, the risk of developing clinical hepatitis from needles contaminated with HBsAg-positive, HBeAg-negative blood is 1%-6%, and the risk of developing serological evidence of HBV infection is 23%-37%20. The current recommendations of the Advisory Committee on Immunization Practices1,2, recommends postexposure prophylaxis with hepatitis B immune globulin and/or hepatitis B vaccine series for any susceptible, unvaccinated person who sustains an occupational blood or body fluid exposure.
The pharmacokinetic profile of HepaGam B has been evaluated in two clinical trials [see Clinical Studies (14.2) ]. The comparative bioavailability of HepaGam B and another commercially available hepatitis B immunoglobulin product indicates that HepaGam B has similar efficacy.
13 NONCLINICAL TOXICOLOGY
Nonclinical pharmacology studies have not been performed with Hepatitis B Immune Globulin Intravenous (Human) as there is broad experience in humans with intravenous and intramuscular administration of immune globulin products. Since the product is of human origin, immunogenicity is expected when administered to animals.
Toxicology studies have not been performed with Hepatitis B Immune Globulin Intravenous (Human) because the product has been formulated with ingredients that are know to be non-toxic at the levels at which they are present in the final product.
14 CLINICAL STUDIES
14.1 Clinical Trials in Liver Transplant Patients
A clinical trial examining the effectiveness of HepaGam B in the prevention of hepatitis B recurrence following liver transplantation is currently ongoing. The study is a multi-center, open-labeled, superiority study involving HBsAg-positive/HBeAg-negative liver transplant patients. The study included two arms, an active treatment group of patients enrolled to receive the described dosing regimen of HepaGam B starting during transplant and continuing over the course of a year, and a retrospective untreated control group of historical patients with data gathered by chart review.
An interim report of this study evaluated the data from 30 liver transplant patients, 16 HepaGam B patients who have completed the study and 14 retrospective untreated control patients. The patients in both groups were HBsAg-positive/HBeAg-negative liver transplant patients who met similar entry criteria, had similar medical history and had similar status at transplant based on MELD and/or ChildPugh-Turcotte scores.
In the active treatment group, HepaGam B doses of 35 mL started during transplant according to the regimen identified in Table 1 [see Dosing and Administration (2.1) ]. As a result of the targeted potency of 550 IU/mL at the time of manufacture [see Dosage Forms and Strengths (3.) ], the 35 mL doses of HepaGam B used in this study actually contained between 17,000 and 23,000 IU anti-HBs. These 35 mL doses consistently yielded anti-HBs trough levels > 500 IU/L (99% of all anti-HBs levels were > 500 IU/L; see Figure 1).
Figure 1: Frequency Histogram of Trough anti-HBs Levels more than 30 days after Transplant
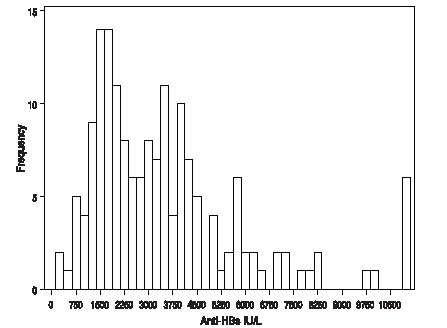
Values below the target trough were only observed in the 2 patients with HBV recurrence who had anti-HBs levels <150 IU/L at the time of seroconversion.
For the efficacy endpoint of the proportion of patients with HBV recurrence (HBsAg positive and/or HBeAg positive after 4 weeks post-OLT), a significant treatment effect was observed. As summarized in Table 6, HBV recurrence was seen in 2/15 or 13% of HepaGam B patients compared to 12/14 or 86% of retrospective untreated control patients (see Table 6). One of the 16 HepaGam B patients died 2 weeks post-transplant was excluded from all efficacy analyses, but was included for safety analyses. The death was not HBV or study drug related.
| HepaGam B | Retrospective Untreated Control |
P-value (Fisher's Exact Test) |
|
|
HBV Recurrence
Proportion, % (95% confidence interval) |
13.3 (1.7 -40.5) |
85.7 (57.2 -98.2) |
< 0.001 |
The conclusion that HepaGam B monotherapy post-OLT is effective at preventing HBV recurrence post-OLT is further supported by the secondary endpoints of time to recurrence, survival, anti-HBs levels and biochemical markers of liver inflammation. Time to recurrence for the HepaGam B treatment group was 358 days for two HBV recurrent patients. In comparison, the retrospective untreated control patients had a median time to recurrence of 88 days with a 95% confidence interval of 47 to 125 days. Survival calculations showed that 93% (14/15) of patients in the active treatment group survived for at least 1-year post-OLT compared to 43% (6/14) retrospective control patients. One patient in the active treatment group died 266 days post-OLT. The median time to death for the retrospective control patients was 339 days calculated using the product limit method. The endpoints for HBV recurrence were supported by an observed drop in anti-HBs levels and elevated liver function tests at the time of recurrence.
HepaGam B is recommended in patients who have no or low levels of viral replication at the time of liver transplantation. The clinical trial evaluating HepaGam B in liver transplant patients selected patients with no or low replication status only. HepaGam B therapy has not been evaluated in combination with antiviral therapy post-transplantation.
14.2 Comparative Bioavailability Studies
The pharmacokinetic profile of HepaGam B after intramuscular injection of 0.06 mL/kg in two 84-day pharmacokinetics studies8, 70 volunteers were administered HepaGam B. The mean peak concentrations (Cmax) in both studies were comparable and occurred within 4-5 days of administration. Both studies demonstrated mean elimination half-lives (t½) following IM administration of 22 to 25 days. The mean clearance rate was 0.21 to 0.24 L/day and the volume of distribution was approximately 7.5 L. Thus, HepaGam B demonstrates pharmacokinetic parameters similar to those reported by Scheiermann and Kuwert21.
The maximum concentration of anti-HBs achieved by HepaGam B was consistent with that of two other licensed Hepatitis B Immune Globulin (Human) products when compared in the two pharmacokinetic trials8. Comparability of pharmacokinetics between HepaGam B and a commercially available hepatitis B immune globulin product administered i.m. indicates that similar efficacy of HepaGam B should be inferred.
15 REFERENCES
- CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Part 1: Immunization of infants, children, and adolescents. MMWR 2005; 54(RR-16): 1-32.
- CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Part 2: Immunization of adults. MMWR 2006; 55(RR-16): 1-33.
- Terrault NA, Zhou S, Combs C, Hahn JA, Lake JR, Roberts JP et al. Prophylaxis in liver transplant recipients using a fixed dosing schedule of hepatitis B immunoglobulin. Hepatology 1996; 24(6):1327-33.
- McGory RW, Ishitani MB, Oliveira WM, Stevenson WC, McCullough CS, Dickson RC et al. Improved outcome of orthotopic liver transplantation for chronic hepatitis B cirrhosis with aggressive passive immunization. Transplantation 1996; 61(9):1358-1364.
- Samuel D, Muller R, Alexander G, Fassati L, Ducot B, Benhamou JP et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med 1993; 329(25):1842-1847.
- Beasley RP et al.: Efficacy of hepatitis B immune globulin for the prevention of perinatal transmission of the hepatitis B virus carrier state: Final report of a randomized double-blind, placebo-controlled trial. Hepatology 1983; 3:135-41.
- Committee for Proprietary Medicinal Products (CPMP). Core SPC for human plasma derived hepatitis-B immunoglobulin for intravenous use (CPMP/BPWG/4027/02). London, UK: The European Agency for the Evaluation of Medicinal Products. 2003.
- Unpublished data on file.
- Bowman JM, et al. WinRho: Rh immune globulin prepared by ion exchange for intravenous use. Canadian Med Assoc J 1980; 123:1121-5.
- Friesen AD, et al. Column ion-exchange preparation and characterization of an Rh immune globulin (WinRho) for intravenous use. Journal Appl Biochem 1981; 3:164-75.
- Horowitz B. Investigations into the application of tri(n-butyl)phosphate /detergent mixtures to blood derivatives. Morgenthaler J (ed): Virus Inactivation in Plasma Products, Curr Stud Hematol Blood Transfus 1989; 56:83-96.
- Burnouf T. Value of virus filtration as method for improving the safety of plasma products. Vox Sang 1996; 70:235-6.
- Grady GF, Lee VA. Hepatitis B immune globulin - prevention of hepatitis from accidental exposure among medical personnel. N Engl J Med 1975; 293:1067-70.
- Seeff LB, et al. Type B hepatitis after needle-stick exposure: Prevention with hepatitis B immune globulin. Ann Int Med 1978; 88: 285-93.
- Krugman S, Giles JP. Viral hepatitis, type B (MS-2-strain). Further observations on natural history and prevention. N Engl J Med 1973; 288: 755-60.
- Hoofnagle JH, et al. Passive-active immunity from hepatitis B immune globulin. Ann Int Med 1979; 91:813-8.
- Shouval D, Samuel D. Hepatitis B immune globulin to prevent hepatitis B virus graft reinfection following liver transplantation: a concise review. Hepatology 2000; 32(6):1189-1195.
- Sawyer RG, McGory RW, Gaffey MJ, McCullough CC, Shephard BL, Houlgrave CW et al. Improved clinical outcomes with liver transplantation for hepatitis B-induced chronic liver failure using passive immunization. Ann Surg 1998; 227(6): 841-50.
- Schilling R, Ijaz S, Davidoff M, Lee JY, Locarnini S, Williams R, Naoumov NV. Endocytosis of hepatitis B immune globulin into hepatocytes inhibits the secretion of hepatitis B virus surface antigen and virions. J Virol 2003;77(16):8882-92.
- CDC. Updated U.S. Public Health Service Guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR 2001; 50(RR-11): 1-42.
- Scheiermann N, Kuwert EK. Uptake and elimination of hepatitis B immunoglobulins after intramuscular application in man. Dev Biol Standard 1983; 54:347-55.
16 HOW SUPPLIED/STORAGE AND HANDLING
NDC 53270-0053-1; a carton containing a 1.0 mL single dose vial (>312 IU/mL; measured potency of each lot is stamped on the vial label) and a package insert.
NDC 53270-0054-1; a carton containing a 5.0 mL single dose vial (>312 IU/mL; measured potency of each lot is stamped on the vial label) and a package insert.
Store at 36 to 46 °F (2 to 8 °C). Do not freeze. Do not use after expiration date. Use within 6 hours after the vial has been entered.
17 PATIENT COUNSELING INFORMATION
Patients should be informed that HepaGam B is prepared from human plasma. Products made from human plasma may contain infectious agents such as viruses that can cause disease. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and/or removing certain viruses during manufacturing. Despite these measures, such products can still potentially transmit disease. There is also the possibility that unknown infectious agents may be present in such products.
Individuals known to have severe, potentially life-threatening reaction to human globulin should not receive HepaGam B or any other immune globulin products. Individuals who are deficient in IgA may have the potential for developing IgA antibodies and have severe potentially life threatening allergic reactions. In case of allergic or anaphylactic reaction, the infusion should be stopped immediately. In case of shock, the current medical standards for treatment of shock should be observed.
The maltose contained in HepaGam B can interfere with some types of blood glucose monitoring systems. Only testing systems that are glucose-specific should be used in patients receiving HepaGam B. This inference can result in falsely elevated glucose readings that can lead to untreated hypoglycemia or to inappropriate insulin administration, resulting in life-threatening hypoglycemia.
Principal Display Panel

NDC 53270-0053-1
Hepatitis B Immune Globulin Intravenous (Human)HepaGam B®
1 mL (>312 IU/mL)
NDC 53270-0053-1
Hepatitis B Immune Globulin Intravenous (Human)
HepaGam B®
1 mL (>312 IU/mL)
FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION
Rx only
Principal Display Panel
NDC 53270-0054-1
Hepatitis B Immune Globulin Intravenous (Human)HepaGam B®
5 mL (>312 IU/mL)

NDC 53270-0054-1
Hepatitis B Immune Globulin Intravenous (Human)
HepaGam B®
5 mL (>312 IU/mL)
FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION
Rx only
HepaGam BHepatitis B Immune Globulin (Human) INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
HepaGam BHepatitis B Immune Globulin (Human) INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||