Heparin Sodium
Heparin Sodium
FULL PRESCRIBING INFORMATION: CONTENTS*
- HEPARIN SODIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- HEPARIN SODIUM INDICATIONS AND USAGE
- HEPARIN SODIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- HEPARIN SODIUM ADVERSE REACTIONS
- OVERDOSAGE
- HEPARIN SODIUM DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- RL-0218
- RL-0219
FULL PRESCRIBING INFORMATION
Injection, USP
2000 and 2500 USP Units/mL
Heparin Sodium
ADD-Vantage™ Vial
Rx only
HEPARIN SODIUM DESCRIPTION
Heparin Sodium Injection, USP is a sterile, nonpyrogenic solution of heparin sodium (derived from porcine intestinal mucosa) in water for injection. Each container contains 10000, 12500, 20000 or 25,000 USP Heparin Units; 40 or 80 mg sodium chloride added to render isotonic (see HOW SUPPLIED section for various sizes and strength). May contain sodium hydroxide and/or hydrochloric acid for pH adjustment. pH 6.0 (5.0 to 7.5).
The solution contains no bacteriostat, antimicrobial agent or added buffer and is intended for use only as a single-dose injection. When smaller doses are required, the unused portion should be discarded.
Heparin sodium in the ADD-Vantage™ system is intended for intravenous administration only after dilution.
Heparin Sodium, USP is a heterogenous group of straight-chain anionic mucopolysaccharides, called glycosamino-glycans having anticoagulant properties. Although others may be present, the main sugars occurring in heparin are: (1) α- L-iduronic acid 2-sulfate, (2) 2-deoxy-2-sulfamino-α-D-glucose-6-sulfate, (3) β-D-glucuronic acid, (4) 2-acetamido-2-deoxy-α-D-glucose, and (5) α-L-iduronic acid. These sugars are present in decreasing amounts, usually in the order (2) > (1) > (4) > (3) > (5), and are joined by glycosidic linkages, forming polymers of varying sizes. Heparin is strongly acidic because of its content of covalently linked sulfate and carboxylic acid groups. In heparin sodium, the acidic protons of the sulfate units are partially replaced by sodium ions. The potency is determined by a biological assay using a USP reference standard based on units of heparin activity per milligram.
Structure of Heparin Sodium (representative subunits):

CLINICAL PHARMACOLOGY
Heparin inhibits reactions that lead to the clotting of blood and the formation of fibrin clots both in vitro and in vivo . Heparin acts at multiple sites in the normal coagulation system. Small amounts of heparin in combination with antithrombin III (heparin cofactor) can inhibit thrombosis by inactivating activated Factor X and inhibiting the conversion of prothrombin to thrombin. Once active thrombosis has developed, larger amounts of heparin can inhibit further coagulation by inactivating thrombin and preventing the conversion of fibrinogen to fibrin. Heparin also prevents the formation of a stable fibrin clot in inhibiting the activation of the fibrin stabilizing factor.
Bleeding time is usually unaffected by heparin. Clotting time is prolonged by full therapeutic doses of heparin; in most cases, it is not measurably affected by low doses of heparin.
Peak plasma levels of heparin are achieved 2 to 4 hours following subcutaneous administration, although there are considerable individual variations. Loglinear plots of heparin plasma concentrations with time for a wide range of dose levels are linear which suggests the absence of zero order processes. Liver and the reticuloendothelial system are the site of biotransformation. The biphasic elimination curve, a rapidly declining alpha phase (t½ = 10΄) and after the age of 40 a slower beta phase, indicates uptake in organs. The absence of a relationship between anticoagulant half-life and concentration half-life may reflect factors such as protein binding of heparin.
Heparin does not have fibrinolytic activity; therefore, it will not lyse existing clots.
HEPARIN SODIUM INDICATIONS AND USAGE
Heparin sodium is indicated for:
Atrial fibrillation with embolization:
Treatment of acute and chronic consumption coagulopathies (disseminated intravascular coagulation);
Prevention of clotting in arterial and heart surgery;
Anticoagulant therapy in prophylaxis and treatment of venous thrombosis and its extension;
(In a low-dose regimen) for prevention of postoperative deep venous thrombosis and pulmonary embolism in patients undergoing major abdomino-thoracic surgery or who for other reasons are at risk of developing thromboembolic disease (see DOSAGE AND ADMINISTRATION );
Prophylaxis and treatment of pulmonary embolism;
Prophylaxis and treatment of peripheral arterial embolism.
HEPARIN SODIUM CONTRAINDICATIONS
Heparin sodium should not be used in patients:
With severe thrombocytopenia;
In whom suitable blood coagulation tests — e.g., the whole blood clotting time, partial thromboplastin time, etc. — cannot be performed at appropriate intervals (this contraindication refers to full-dose heparin; there is usually no need to monitor coagulation parameters in patients receiving low-dose heparin);
With an uncontrollable active bleeding state (see WARNINGS ), except when this is due to disseminated intravascular coagulation.
Intravenous solutions with Heparin Sodium Injection are contraindicated in patients who are hypersensitive to heparin.
WARNINGS
Heparin is not intended for intramuscular use.
Fatal Medication Errors
Do not use Heparin Sodium Injection as a “catheter lock flush” product. Heparin Sodium Injection is supplied in vials containing various strengths of heparin, including vials that contain a highly concentrated solution of 10,000 units in 1 mL. Fatal hemorrhages have occurred in pediatric patients due to medication errors in which 1 mL Heparin Sodium Injection vials were confused with 1 mL “catheter lock flush” vials. Carefully examine all Heparin Sodium Injection vials to confirm the correct vial choice prior to administration of the drug.
Hypersensitivity: Patients with documented hypersensitivity to heparin should be given the drug only in clearly life-threatening situations.
Hemorrhage: Hemorrhage can occur at virtually any site in patients receiving heparin. An unexplained fall in hematocrit, fall in blood pressure or any other unexplained symptom should lead to serious consideration of a hemorrhagic event.
Heparin sodium should be used with extreme caution in disease states in which there is increased danger of hemorrhage. Some of the conditions in which increased danger of hemorrhage exists are:
Cardiovascular — Subacute bacterial endocarditis. Severe hypertension.
Surgical — During and immediately following (a) spinal tap or spinal anesthesia or (b) major surgery, especially involving the brain, spinal cord or eye.
Hematologic — Conditions associated with increased bleeding tendencies, such as hemophilia, thrombocytopenia, and some vascular purpuras.
Gastrointestinal — Ulcerative lesions and continuous tube drainage of the stomach or small intestine.
Other — Menstruation, liver disease with impaired hemostasis.
Coagulation Testing: When heparin sodium is administered in therapeutic amounts, its dosage should be regulated by frequent blood coagulation tests. If the coagulation test is unduly prolonged or if hemorrhage occurs, heparin sodium should be discontinued promptly (see OVERDOSAGE ).
Thrombocytopenia: Thrombocytopenia in patients receiving heparin has been reported at frequencies up to 30%. It can occur 2 to 20 days (average 5 to 9) following the onset of heparin therapy. Obtain platelet counts before and periodically during heparin therapy. Monitor thrombocytopenia of any degree closely. If the count falls below 100,000/mm3 or if recurrent thrombosis develops, promptly discontinue heparin, evaluate for HIT and HITT, and, if necessary, administer an alternative anticoagulant (see Heparin-induced Thrombocytopenia and Heparin-induced Thrombocytopenia and Thrombosis ).
Heparin-Induced Thrombocytopenia and Heparin-Induced Thrombocytopenia and Thrombosis: Heparin-induced thrombocytopenia (HIT) is a serious antibody-mediated reaction resulting from irreversible aggregation of platelets. HIT may progress to the development of venous and arterial thromboses, a condition known as heparin-induced thrombocytopenia and thrombosis (HITT).
Thrombotic events may also be the initial presentation for HITT. These serious thromboembolic events include deep vein thrombosis, pulmonary embolism, cerebral vein thrombosis, limb ischemia, stroke, myocardial infarction, mesenteric thrombosis, renal arterial thrombosis, skin necrosis, gangrene of the extremities that may lead to amputation, and possibly death. Monitor thrombocytopenia of any degree closely. If the platelet count falls below 100,000/mm3 or if recurrent thrombosis develops, promptly discontinue heparin, evaluate for HIT and HITT, and, if necessary, administer an alternative anticoagulant.
HIT and HITT can occur up to several weeks after the discontinuation of heparin therapy. Patients presenting with thrombocytopenia or thrombosis after discontinuation of heparin should be evaluated for HIT and HITT.
PRECAUTIONS
General:
Do not administer unless solution is clear and container is intact. Discard unused portion (see DOSAGE AND ADMINISTRATION ).
a. Heparin Resistance:
Increased resistance to heparin is frequently encountered in fever, thrombosis, thrombophlebitis, infections with thrombosing tendencies, myocardial infarction, cancer and in postsurgical patients.
b. Increased Risk in Older Women:
A higher incidence of bleeding has been reported in women over 60 years of age.
Laboratory Tests: Periodic platelet counts, hematocrits and tests for occult blood in stool are recommended during the entire course of heparin therapy (see DOSAGE AND ADMINISTRATION ).
Drug Interactions:
Oral anticoagulants: Heparin sodium may prolong the one-stage prothrombin time. Therefore, when heparin sodium is given with dicumarol or warfarin sodium, a period of at least 5 hours after the last intravenous dose should elapse before blood is drawn if a valid PROTHROMBIN time is to be obtained.
Platelet inhibitors: Drugs such as acetylsalicylic acid, dextran, phenylbutazone, ibuprofen, indomethacin, dipyridamole, hydroxychloroquine and others that interfere with platelet aggregation reactions (the main hemostatic defense of heparinized patients) may induce bleeding and should be used with caution in patients receiving heparin sodium.
Other interactions: Digitalis, tetracyclines, nicotine, antihistamines or I.V. nitroglycerin may partially counteract the anticoagulant action of heparin sodium.
Drug/Laboratory Test Interactions:
Hyperaminotransferasemia: Significant elevations of aminotransferase (SGOT [S-AST] and SGPT [S-ALT]) levels have occurred in a high percentage of patients (and healthy subjects) who have received heparin. Since aminotransferase determinations are important in the differential diagnosis of myocardial infarction, liver disease, and pulmonary emboli, rises that might be caused by drugs (like heparin) should be interpreted with caution.
Carcinogenesis, Mutagenesis, Impairment of Fertility: No long-term studies in animals have been performed to evaluate carcinogenic potential of heparin. Also, no reproduction studies in animals have been performed concerning mutagenesis or impairment of fertility.
Pregnancy:
Pregnancy Category C: There are no adequate and well-controlled studies on heparin use in pregnant women. In published reports, heparin exposure during pregnancy did not show evidence of an increased risk of adverse maternal or fetal outcomes in humans. Heparin sodium does not cross the placenta, based on human and animal studies. Administration of heparin to pregnant animals at doses higher than the maximum human daily dose based on body weight resulted in increased resorptions. Use heparin sodium during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In a published study conducted in rats and rabbits, pregnant animals received heparin intravenously during organogenesis at a dose of 10,000 units/kg/day, approximately 10 times the maximum human daily dose based on body weight. The number of early resorptions increased in both species. There was no evidence of teratogenic effects.
Nursing Mothers: Due to its large molecular weight, heparin is not likely to be excreted in human milk, and any heparin in milk would not be orally absorbed by a nursing infant. Benzyl alcohol present in maternal serum is likely to cross into human milk and may be orally absorbed by a nursing infant. Exercise caution when administering Heparin Sodium Injection to a nursing mother.
Pediatric Use: There are no adequate and well controlled studies on heparin use in pediatric patients. Pediatric dosingrecommendations are based on clinical experience (see DOSAGE AND ADMINISTRATION , Pediatric Use ).
Carefully examine all Heparin Sodium Injection vials to confirm choice of the correct strength prior to administration of the drug. Pediatric patients, including neonates, have died as a result of medication errors in which HEPARIN SODIUM INJECTION vials have been confused with “catheter lock flush” vials (see WARNINGS, Fatal Medication Errors ).
HEPARIN SODIUM ADVERSE REACTIONS
Hemorrhage: Hemorrhage is the chief complication that may result from heparin therapy (see WARNINGS ). An overly prolonged clotting time or minor bleeding during therapy can usually be controlled by withdrawing the drug (see OVERDOSAGE ). It should be appreciated that gastrointestinal or urinary tract bleeding during anticoagulant therapy may indicate the presence of an underlying occult lesion. Bleeding can occur at any site but certain specific hemorrhagic complications may be difficult to detect:
a. Adrenal hemorrhage, with resultant acute adrenal insufficiency, has occurred during anticoagulant therapy. Therefore, such treatment should be discontinued in patients who develop signs and symptoms of acute adrenal hemorrhage and insufficiency. Initiation of corrective therapy should not depend on laboratory confirmation of the diagnosis since any delay in an acute situation may result in the patient’s death.
b. Ovarian (corpus luteum) hemorrhage developed in a number of women of reproductive age receiving short- or long-term anticoagulant therapy. This complication if unrecognized may be fatal.
c. Retroperitoneal hemorrhage.
Hypersensitivity: Generalized hypersensitivity reactions have been reported with chills, fever, and urticaria as the most usual manifestations, and asthma, rhinitis, lacrimation, headache, nausea and vomiting and anaphylactoid reactions, including shock, occurring more rarely. Itching and burning, especially on the plantar site of the feet may occur.
Thrombocytopenia has been reported to occur in patients receiving heparin with a reported incidence of 0 to 30%. While often mild and of no obvious clinical significance, such thrombocytopenia can be accompanied by severe thromboembolic complications such as skin necrosis, gangrene of the extremities that may lead to amputation, myocardial infarction, pulmonary embolism, stroke and possibly death. (See WARNINGS and PRECAUTION S.)
Certain episodes of painful, ischemic and cyanosed limbs have in the past been attributed to allergic vasospastic reactions. Whether these are in fact identical to the thrombocytopenia associated complications remains to be determined.
Miscellaneous: Osteoporosis following long-term administration of high doses of heparin, cutaneous necrosis after systemic administration, suppression of aldosterone synthesis, delayed transient alopecia, priapism and rebound hyperlipemia on discontinuation of heparin sodium have also been reported.
Significant elevations of aminotransferase (SGOT [S-AST] and SGPT [S-ALT]) levels have occurred in a high percentage of patients (and healthy subjects) who have received heparin.
OVERDOSAGE
Symptoms: Bleeding is the chief sign of heparin overdosage. Nosebleeds, blood in urine or tarry stools may be noted as the first sign of bleeding. Easy bruising or petechial formations may precede frank bleeding.
Treatment: Neutralization of heparin effect.
When clinical circumstances (bleeding) require reversal of heparinization, protamine sulfate (1% solution) by slow infusion will neutralize heparin sodium. No more than 50 mg should be administered, very slowly in any 10 minute period. Each mg of protamine sulfate neutralizes approximately 100 USP heparin units. The amount of protamine required decreases over time as heparin is metabolized. Although the metabolism of heparin is complex, it may, for the purpose of choosing a protamine dose, be assumed to have a half-life of about ½ hour after intravenous injection.
Administration of protamine sulfate can cause severe hypotensive and anaphylactoid reactions. Because fatal reactions often resembling anaphylaxis have been reported, the drug should be given only when resuscitation techniques and treatment of anaphylactoid shock are readily available.
For additional information, the labeling of Protamine Sulfate Injection, USP products should be consulted.
HEPARIN SODIUM DOSAGE AND ADMINISTRATION
Heparin sodium is not effective by oral administration and should be given by intermittent intravenous injection, after dilution in 50 or 100 mL of 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP, or by intravenous infusion.
The dosage of heparin sodium should be adjusted according to the patient’s coagulation test results. When heparin is given by continuous intravenous infusion, the coagulation time should be determined approximately every 4 hours in the early stages of treatment. When the drug is administered intermittently by intravenous injection, coagulation tests should be performed before each injection during the early stages of treatment and at appropriate intervals thereafter. Dosage is considered adequate when the activated partial thromboplastin time (APTT) is 1.5 to 2 times normal or when the whole blood clotting time is elevated approximately 2.5 to 3 times the control value.
Periodic platelet counts, hematocrits and tests for occult blood in stool are recommended during the entire course of heparin therapy.
Converting to Oral Anticoagulant: When an oral anticoagulant of the coumarin or similar type is to be begun in patients already receiving heparin sodium, baseline and subsequent tests of prothrombin activity must be determined at a time when heparin activity is too low to affect the prothrombin time. If continuous I.V. heparin infusion is used, prothrombin time can usually be measured at any time.
In converting from heparin to an oral anticoagulant, the dose of the oral anticoagulant should be the usual initial amount and thereafter prothrombin time should be determined at the usual intervals. To ensure continuous anticoagulation, it is advisable to continue full heparin therapy for several days after the prothrombin time has reached the therapeutic range. Heparin therapy may then be discontinued without tapering.
Therapeutic Anticoagulant Effect with Full-Dose Heparin. Although dosage must be adjusted for the individual patient according to the results of suitable laboratory tests, the following dosage schedules may be used as guidelines:
|
Method of Administration |
Frequency |
Recommended dose* |
|
Intermittent Intravenous Injection |
Initial Dose |
10,000 Units, in 50—100 mL of 5% Dextrose Injection or 0.9% Sodium Chloride Injection |
|
Every 4 to 6 hours |
5000—10,000 Units, in 50—100 mL of 5% Dextrose Injection or 0.9% Sodium Chloride Injection |
|
|
Continuous Intravenous Infusion |
Initial Dose |
5000 Units by I.V. Injection |
|
Continuous |
20,000—40,000 Units/24 hours in 5% Dextrose Injection or 0.9% Sodium Chloride Injection |
|
|
* Based on 150 lb. (68 kg) patient. |
||
Pediatric Use: There are no adequate and well controlled studies on heparin use in pediatric patients. Pediatric dosing recommendations are based on clinical experience. In general, the following dosage schedule may be used as a guideline in pediatric patients:
Initial Dose: 75 to 100 units/kg (IV bolus over 10 minutes)
Maintenance Dose Infants: 25 to 30 units/kg/hour;
Infants < 2 months have the highest requirements (average 28 units/kg/hour)
Children > 1 year of age: 18 to 20 units/kg/hour;
Older children may require less heparin, similar to weight-adjusted adult dosage
Monitoring: Adjust heparin to maintain a PTT of 60 to 85 seconds, assuming this reflects an anti-Factor Xa level of 0.35 to 0.70.
Surgery of the Heart and Blood Vessels: Patients undergoing total body perfusion for open-heart surgery should receive an initial dose of not less than 150 units of heparin sodium per kilogram of body weight. Frequently, a dose of 300 units per kilogram is used for procedures estimated to last less than 60 minutes or 400 units per kilogram for those estimated to last longer than 60 minutes.
Low-Dose Prophylaxis of Postoperative Thromboembolism: A number of well-controlled clinical trials have demonstrated that low-dose heparin prophylaxis, given just prior to and after surgery, will reduce the incidence of postoperative deep vein thrombosis in the legs (as measured by the I-125 fibrinogen technique and venography) and of clinical pulmonary embolism. The most widely used dosage has been 5,000 units 2 hours before surgery and 5,000 units every 8 to 12 hours thereafter for 7 days or until the patient is fully ambulatory, whichever is longer. A concentrated solution of heparin sodium is recommended. Such prophylaxis should be reserved for patients over the age of 40 who are undergoing major surgery. Patients with bleeding disorders and those having brain or spinal cord surgery, spinal anesthesia, eye surgery, or potentially sanguineous operations should be excluded, as should patients receiving oral anticoagulants or platelet-active drugs (see WARNINGS ). The value of such prophylaxis in hip surgery has not been established. The possibility of increased bleeding during surgery or postoperatively should be borne in mind. If such bleeding occurs, discontinuance of heparin and neutralization with protamine sulfate are advisable. If clinical evidence of thromboembolism develops despite low-dose prophylaxis, full therapeutic doses of anticoagulants should be given unless contraindicated. Prior to initiating heparinization the physician should rule out bleeding disorders by appropriate history and laboratory tests, and appropriate coagulation tests should be repeated just prior to surgery. Coagulation test values should be normal or only slightly elevated at these times.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Slight discoloration does not alter potency. See PRECAUTIONS .
INSTRUCTIONS FOR USE
To Open Diluent Container:
Peel overwrap from the corner and remove container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Assemble Vial and Flexible Diluent Container:
(Use Aseptic Technique)
-
Remove the protective covers from the top of the vial and the vial port on the diluent container as follows:
a. To remove the breakaway vial cap, swing the pull ring over the top of the vial and pull down far enough to start the opening (see Figure 1.), then pull straight up to remove the cap. (see Figure 2.) NOTE: Once the breakaway cap has been removed, do not access vial with syringe.
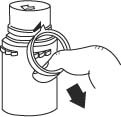

Fig. 1
Fig. 2
b. To remove the vial port cover, grasp the tab on the pull ring, pull up to break the three tie strings, then pull back to remove the cover. (see Figure 3.)
-
Screw the vial into the vial port until it will go no further. THE VIAL MUST BE SCREWED IN TIGHTLY TO ASSURE A SEAL. This occurs approximately ½ turn (180°) after the first audible click. (see Figure 4.) The clicking sound does not assure a seal; the vial must be turned as far as it will go. NOTE: Once vial is seated, do not attempt to remove. (see Figure 4.)
-
Recheck the vial to assure that it is tight by trying to turn it further in the direction of assembly.
-
Label appropriately.


Fig. 3
Fig. 4
To Prepare Admixture:
-
Squeeze the bottom of the diluent container gently to inflate the portion of the container surrounding the end of the drug vial.
-
With the other hand, push the drug vial down into the container telescoping the walls of the container. Grasp the inner cap of the vial through the walls of the container. (see Figure 5.)
-
Pull the inner cap from the drug vial. (see Figure 6.) Verify that the rubber stopper has been pulled out, allowing the drug and diluent to mix.
-
Mix container contents thoroughly and use within the specified time.
|
|
|
|
Fig. 5 |
Fig. 6 |
Preparation for Administration
(Use Aseptic Technique)
-
Confirm the activation and admixture of vial contents.
-
Check for leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired.
-
Close flow control clamp of administration set.
-
Remove cover from outlet port at bottom of container.
-
Insert piercing pin of administration set into port with a twisting motion until the pin is firmly seated. NOTE: See full directions on administration set carton.
-
Lift the free end of the hanger loop on the bottom of the vial, breaking the two tie strings. Bend the loop outward to lock it in the upright position, then suspend container from hanger.
-
Squeeze and release drip chamber to establish proper fluid level in chamber.
-
Open flow control clamp and clear air from set. Close clamp.
-
Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
-
Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible container in series connections.
HOW SUPPLIED
Heparin Sodium Injection, USP is supplied in the ADD-Vantage™ vials as follows:
|
NDC No. |
Total Volume |
Total Units Heparin/vial |
Total mg Sodium Chloride/vial |
|
0409-2581-02 |
5 mL |
10,000 |
40 |
|
0409-2584-02 |
10 mL |
25,000 |
80 |
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Caution: Federal (USA) law prohibits dispensing without prescription.
Revised: October, 2011
Printed in USA EN-2899
Hospira, Inc., Lake Forest, IL 60045 USA
RL-0218

RL-0219

Heparin SodiumHEPARIN SODIUM INJECTION, SOLUTION, CONCENTRATE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Heparin SodiumHEPARIN SODIUM INJECTION, SOLUTION, CONCENTRATE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

