HUMCO Mercuroclear
HUMCO Mercuroclear
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- active Ingredient
- Purpose
- HUMCO Mercuroclear Uses
- Warnings
- Ask a doctor before use if you have
- When using this product
- Stop use and aska doctor if
- Directions
- Other Information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
- Principal Display Panel
FULL PRESCRIBING INFORMATION
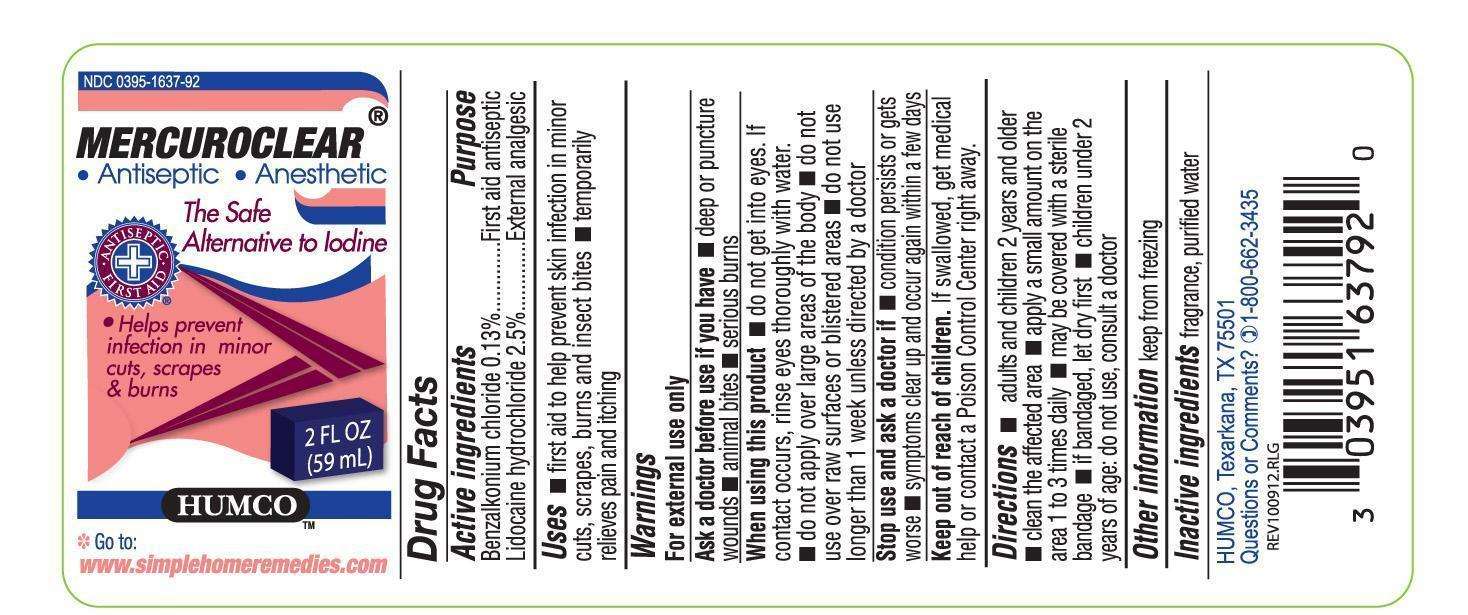
Drug Facts
Active Ingredient
Benzalkonium chloride 0.13%
Purpose
First Aid Antiseptic
active Ingredient
Lidocaine hydrochloride 2.5%
Purpose
External analgesic
HUMCO Mercuroclear Uses
first aid to help prevent skin infection in minor cuts, scrapes, burns and insect bites. Temporary relieves pain and itching.
Warnings
For external use only
Ask a doctor before use if you have
deep or puncture wounds, animal bites, serious burns.
When using this product
do not get into eyes. If contact occurs, rinse eyesthroughly with water. do not apply over large areas of the body. do not use over raw surfaces or blistered areas. do not use longer than 1 week unless directed by a doctor.
Stop use and aska doctor if
condition persists or gets worse. symptoms clear up and occur again within a few days.
Directions
adults and children 2 years and older. clean the affected area. apply a small amount on the area 1 to 3 times daily. may be covered with a sterile bandage. if bandaged, let it dry first. children under 2 years of age, do not use, consult a doctor.
Other Information
Keep from freezing
Inactive ingredients
fragrance, purified water
HUMCO, Texarkana TX 75501
Questions or comments?
1-800-662-3435
Principal Display Panel
NDC 0395-1637-92
Mercuroclear(r)
Antiseptic. Anesthetic
The Safe Alternative to Iodine
Helps prevent infection in minor cuts, scrapes & burns
2 FL OZ (59 mL)
HUMCO
Go to:
Principal Display Panel
NDC 0395-1637-93
Mercuroclear(r)
Antiseptic. Anesthetic
The Safe Alternative to Iodine
Helps prevent infection in minor cuts, scrapes & burns
3 FL OZ (90 mL)
HUMCO
Go to: www.simplehomeremedies.com

HUMCO MercuroclearBenzalkonium Chloride and Lidocaine Hydrochloride LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||