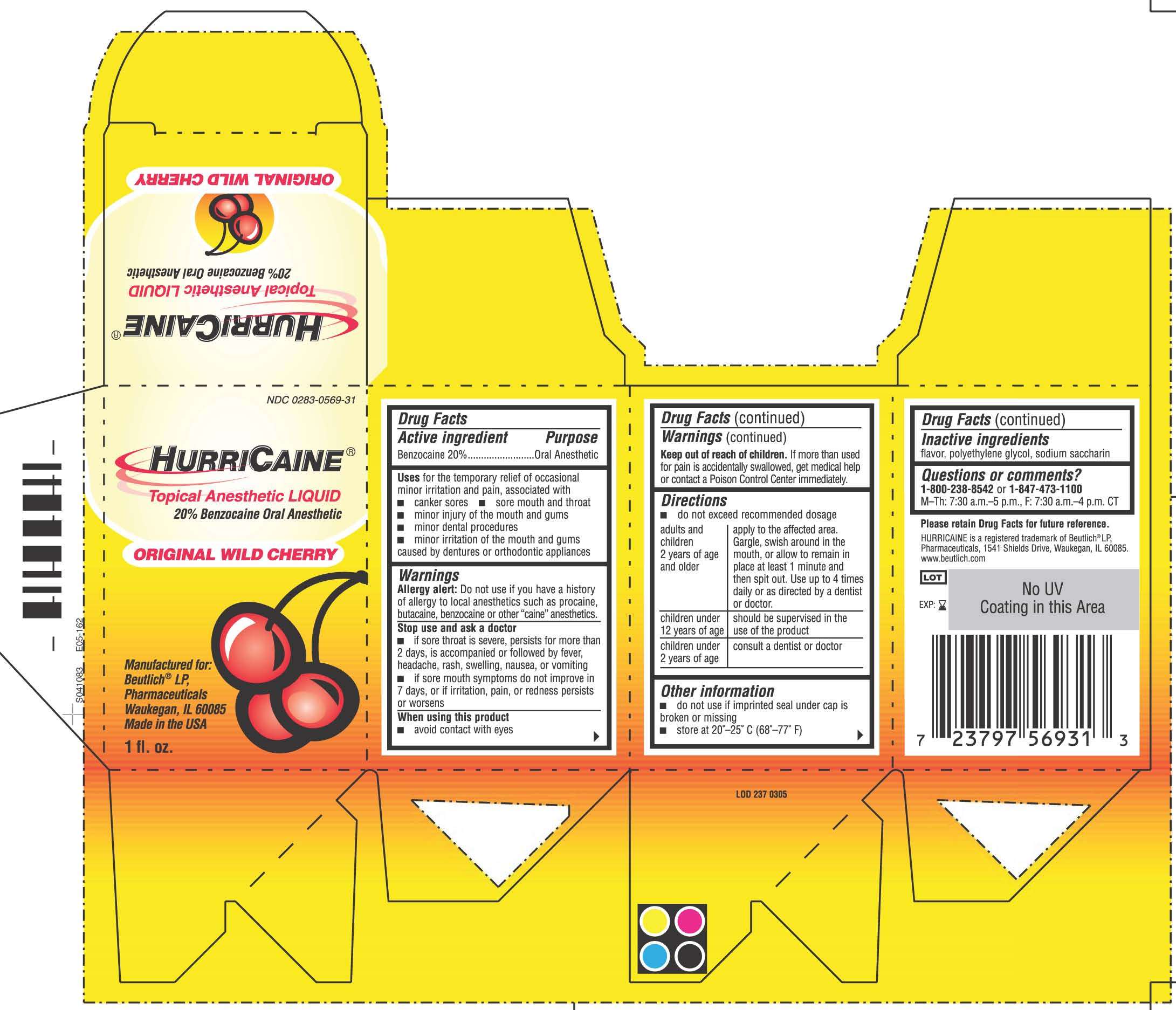
HurriCaine
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- HurriCaine Uses
- Warnings
- Stop use and ask a doctor
- When using this product
- Keep out of reach of children
- Directions
- HurriCaine Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Benzocaine 20%
Purpose
Oral Anesthetic
HurriCaine Uses
for temporary relief of occasional minor irritation and pain associated with:
- canker sores
- sore mouth and throat
- minor injury of the mouth and gums
- minor dental procedures
- minor irritation of the mouth and gums caused by dentures or orthodontic appliances
Warnings
Allergy alert: Do not use if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
Stop use and ask a doctor
- if sore throat is severe, persists for more than 2 days, is accompanied or followed by a fever, headache, rash, swelling, nausea or vomiting
- if sore mouth symptoms do not improve in 7 days, or irritation, pain, or redness persists or worsens
When using this product
- avoid contact with eyes
Keep out of reach of children
If more than used for pain is accidentally swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- do not exceed recommended dosage
HurriCaine Other information
- Do not use if imprinted seal under cap is broken or missing
- store at 20°-25°C (68°-77°F)
Inactive ingredients
flavor, polyethylene glycol, sodium saccharin
Questions or comments?
1-800-238-8542 or 1-847-473-1100
M-Th: 7:30 a.m.-5 p.m., F: 7:30 a.m.-4 p.m. CT
Principal Display Panel
NDC 0283-0569-31
HURRICAINE®
Topical Anesthetic LIQUID
20% Benzocaine Oral Anesthetic
ORIGINAL WILD CHERRY
Manufactured for:
Beutlich® LP,
Pharmaceuticals
Waukegan, IL 60085
Made in the USA
1 fl. oz.
HurriCaineBenzocaine LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||