Hydralazine Hydrochloride
HydrALAZINE Hydrochloride Tablets, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDRALAZINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- HYDRALAZINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- HYDRALAZINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
HYDRALAZINE HYDROCHLORIDE DESCRIPTION
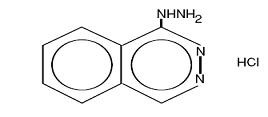
884
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
HYDRALAZINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
See PRECAUTIONS, Laboratory Tests.
PRECAUTIONS
General
Information for Patients
Laboratory Tests
Drug/Drug Interactions
MAO inhibitors should be used with caution in patients receiving HydrALAZINE .
Drug/Food Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a lifetime study in Swiss albino mice, there was a statistically significant increase in the incidence of lung tumors (adenomas and adenocarcinomas) of both male and female mice given HydrALAZINE continuously in their drinking water at a dosage of about 250 mg/kg per day (about 80 times the maximum recommended human dose). In a 2-year carcinogenicity study of rats given HydrALAZINE by gavage at dose levels of 15, 30, and 60 mg/kg/day (approximately 5 to 20 times the recommended human daily dosage), microscopic examination of the liver revealed a small, but statistically significant, increase in benign neoplastic nodules in male and female rats from the high-dose group and in female rats from the intermediate-dose group. Benign interstitial cell tumors of the testes were also significantly increased in male rats from the high-dose group. The tumors observed are common in aged rats and a significantly increased incidence was not observed until 18 months of treatment. HydrALAZINE was shown to be mutagenic in bacterial systems (Gene Mutation and DNA Repair) and in one of two rats and one rabbit hepatocyte in vitro DNA repair studies. Additional in vivo and in vitro studies using lymphoma cells, germinal cells, and fibroblasts from mice, bone marrow cells from chinese hamsters and fibroblasts from human cell lines did not demonstrate any mutagenic potential for HydrALAZINE .
Pregnancy Category C
Nursing Mothers
Pediatric Use
HYDRALAZINE HYDROCHLORIDE ADVERSE REACTIONS
Common:
Less Frequent:Digestive:
Cardiovascular:
Respiratory:
Neurologic:
Genitourinary:
Hematologic:
Hypersensitive Reactions:
Other:
OVERDOSAGE
Acute Toxicity
50
Signs and Symptoms
Treatment
The gastric contents should be evacuated, taking adequate precautions against aspiration and for protection of the airway. An activated charcoal slurry may be instilled if conditions permit. These manipulations may have to be omitted or carried out after cardiovascular status has been stabilized, since they might precipitate cardiac arrhythmias or increase the depth of shock.
Support of the cardiovascular system is of primary importance. Shock should be treated with plasma expanders. If possible, vasopressors should not be given, but if a vasopressor is required, care should be taken not to precipitate or aggravate cardiac arrhythmia.
DOSAGE & ADMINISTRATION
Initiate therapy in gradually increasing dosages; adjust according to individual response. Start with 10 mg four times daily for the first 2 to 4 days, increase to 25 mg four times daily for the balance of the first week. For the second and subsequent weeks, increase dosage to 50 mg four times daily. For maintenance, adjust dosage to the lowest effective levels.
HOW SUPPLIED
| 10 mg | Orange colored, slightly mosaic, circular, biconvex uncoated tablets debossed with ‘293’ on one side and plain on other side Bottles of 100 Tablets NDC 67877-293-01 Bottles of 500 Tablets NDC 67877-293-05 Bottles of 1000 Tablets NDC 67877-293-10 |
| 25 mg | Orange colored, slightly mosaic, circular, biconvex uncoated tablets debossed with ‘292’ on one side and plain on other side Bottles of 100 Tablets NDC 67877-292-01 Bottles of 500 Tablets NDC 67877-292-05 Bottles of 1000 Tablets NDC 67877-292-10 |
| 50 mg | Orange colored, slightly mosaic, circular, biconvex uncoated tablets debossed with ‘291’ on one side and plain on other side Bottles of 100 Tablets NDC 67877-291-01 Bottles of 500 Tablets NDC 67877-291-05 Bottles of 1000 Tablets NDC 67877-291-10 |
| 100 mg | Orange colored, slightly mosaic, circular, biconvex uncoated tablets debossed with ‘290’ on one side and plain on other side Bottles of 100 Tablets NDC 67877-290-01 Bottles of 500 Tablets NDC 67877-290-05 |
Manufactured by:
ALKEM LABORATORIES LIMITED
Distributed by:
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
HydrALAZINE Hydrochloride Tablets, USP 10 mg - Container Label
NDC 67877-293-01
HydrALAZINE
Hydrochloride
Tablets, USP
10 mg
Rx Only
100 Tablets

HydrALAZINE Hydrochloride Tablets, USP 25 mg - Container Label
NDC 67877-292-05
HydrALAZINE
Hydrochloride
Tablets, USP
25 mg
Rx Only
500 Tablets
HydrALAZINE Hydrochloride Tablets, USP 50 mg - Container Label
NDC 67877-291-10
HydrALAZINE
Hydrochloride
Tablets, USP
50 mg
Rx Only
1000 Tablets

HydrALAZINE Hydrochloride Tablets, USP 100 mg - Container Label
NDC 67877-290-01
HydrALAZINE
Hydrochloride
Tablets, USP
100 mg
Rx Only
100 Tablets

Hydralazine HydrochlorideHydralazine Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Hydralazine HydrochlorideHydralazine Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Hydralazine HydrochlorideHydralazine Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Hydralazine HydrochlorideHydralazine Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||