Hydrochlorothiazide
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDROCHLOROTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
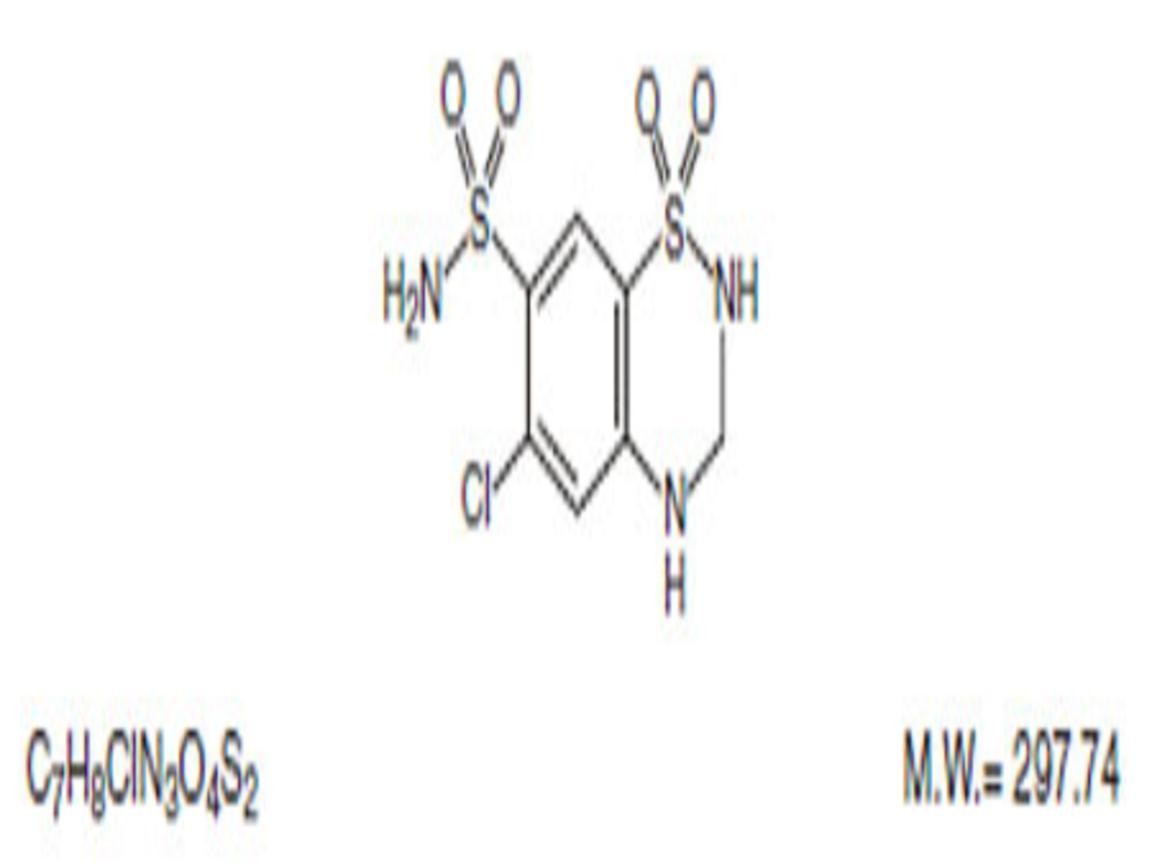
HYDROCHLOROTHIAZIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Pharmacokinetics and Metabolism
INDICATIONS & USAGE
Use in Pregnancy
PRECAUTIONS, Pregnancy
HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS, Drug Interactions
Acute Myopia and Secondary Angle-Closure Glaucoma
PRECAUTIONS
GeneralLABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
PRECAUTIONS, GeneralCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category B
Nonteratogenic Effects
NURSING MOTHERS
PEDIATRIC USE
DOSAGE AND ADMINISTRATION, Infants and ChildrenHYDROCHLOROTHIAZIDE ADVERSE REACTIONS
PRECAUTIONS
WARNINGS
OVERDOSAGE
DOSAGE & ADMINISTRATION
Adults
For Edema
For Control of Hypertension
PRECAUTIONS
Infants and Children
For Diuresis and For Control of Hypertension
PRECAUTIONS, Pediatric Use
HOW SUPPLIED
-
● Bottles of 10: NDC 0603-3856-10
-
● Bottles of 30: NDC 0603-3856-16
-
● Bottles of 50: NDC 0603-3856-19
-
● Bottles of 100: NDC 0603-3856-21
-
● Bottles of 500: NDC 0603-3856-28
-
● Bottles of 1000: NDC 0603-3856-32
-
● Bottles of 5000: NDC 0603-3856-34
-
● Hydrochlorothiazide Tablets, USP 50 mg, are peach-colored, round, scored tablets debossed "3572" and "V" on one side.
-
● Bottles of 30: NDC 0603-3857-16
-
● Bottles of 50: NDC 0603-3857-19
-
● Bottles of 100: NDC 0603-3857-21
-
● Bottles of 500: NDC 0603-3857-28
-
● Bottles of 1000: NDC 0603-3857-32
-
●
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

HydrochlorothiazideHydrochlorothiazide TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!