Hydrocodone Bitartrate and Acetaminophen
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
WARNING
HepatotoxicityAcetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product.HYDROCODONE BITARTRATE AND ACETAMINOPHEN DESCRIPTION
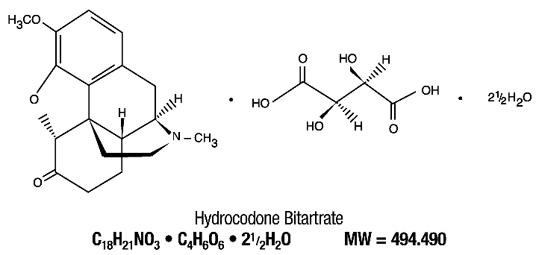
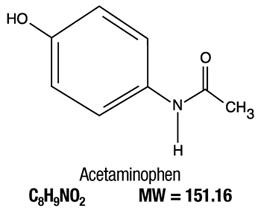
Tablet Hydrocodone Bitartrate USP Acetaminophen USP
CLINICAL PHARMACOLOGY
PharmacokineticsThe behavior of the individual components is described below.
HydrocodoneFollowing a 10 mg oral dose of hydrocodone administered to five adult male subjects, the mean peak concentration was 23.65.2 ng/mL. Maximum serum levels were achieved at 1.30.3 hours and the half-life was determined to be 3.80.3 hours. Hydrocodone exhibits a complex pattern of metabolism including O-demethylation, N-demethylation and 6-keto reduction to the corresponding 6-and 6-(seeOVERDOSAGEfor toxicity information).
AcetaminophenAcetaminophen is rapidly absorbed from the gastrointestinal tract and is distributed throughout most body tissues. The plasma half-life is 1.25 to 3 hours, but may be increased by liver damage and following overdosage. Elimination of acetaminophen is principally by liver metabolism (conjugation) and subsequent renal excretion of metabolites. Approximately 85% of an oral dose appears in the urine within 24 hours of administration, most as the glucuronide conjugate, with small amounts of other conjugates and unchanged drug (seeOVERDOSAGEfor toxicity information).
INDICATIONS & USAGE
HYDROCODONE BITARTRATE AND ACETAMINOPHEN CONTRAINDICATIONS
WARNINGS
HepatotoxicityAcetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product. The excessive intake of acetaminophen may be intentional to cause self-harm or unintentional as patients attempt to obtain more pain relief or unknowingly take other acetaminophen-containing products.Hypersensitivity/anaphylaxisThere have been post-marketing reports of hypersensitivity and anaphylaxis associated with use of acetaminophen. Clinical signs included swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, pruritus, and vomiting. There were infrequent reports of life-threatening anaphylaxis requiring emergency medical attention. Instruct patients to discontinue Hydrocodone Bitartrate and Acetaminophen Tablets USP immediately and seek medical care if they experience these symptoms. Do not prescribe Hydrocodone Bitartrate and Acetaminophen Tablets USP for patients with acetaminophen allergy.
Respiratory DepressionAt high doses or in sensitive patients, hydrocodone may produce dose-related respiratory depression by acting directly on the brain stem respiratory center. Hydrocodone also affects the center that controls respiratory rhythm, and may produce irregular and periodic breathing.
Head Injury and Increased Intracranial PressureThe respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a preexisting increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries.
Acute Abdominal ConditionsThe administration of narcotics may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
Misuse, Abuse, and Diversion of OpioidsHydrocodone bitartrate and acetaminophen tablets contains hydrocodone an opioid agonist, and is a Schedule III controlled substance. Opioid agonists have the potential for being abused and are sought by abusers and people with addiction disorders, and are subject to diversion.
DRUG ABUSE AND DEPENDENCE).
PRECAUTIONS
GeneralSpecial Risk PatientsAs with any narcotic analgesic agent, hydrocodone bitartrate and acetaminophen tablets should be used with caution in elderly or debilitated patients and those with severe impairment of hepatic or renal function, hypothyroidism, Addison's disease, prostatic hypertrophy or urethral stricture. The usual precautions should be observed and the possibility of respiratory depression should be kept in mind.
Cough ReflexHydrocodone suppresses the cough reflex; as with all narcotics, caution should be exercised when hydrocodone bitartrate and acetaminophen tablets are used postoperatively and in patients with pulmonary disease.
Information for Patients/Caregivers
Laboratory TestsIn patients with severe hepatic or renal disease, effects of therapy should be monitored with serial liver and/or renal function tests.
Drug InteractionsPatients receiving other narcotic analgesics, antihistamines, antipsychotics, antianxiety agents, or other CNS depressants (including alcohol) concomitantly with hydrocodone bitartrate and acetaminophen tablets may exhibit an additive CNS depression. When combined therapy is contemplated, the dose of one or both agents should be reduced.
Drug/Laboratory Test InteractionsAcetaminophen may produce false-positive test results for urinary 5-hydroxyindoleacetic acid.
Carcinogenesis, Mutagenesis, Impairment of FertilityNo adequate studies have been conducted in animals to determine whether hydrocodone or acetaminophen have a potential for carcinogenesis, mutagenesis, or impairment of fertility.
Pregnancy
Teratogenic Effects.Pregnancy Category CThere are no adequate and well-controlled studies in pregnant women. Hydrocodone bitartrate and acetaminophen tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic EffectsBabies born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose. There is no consensus on the best method of managing withdrawal.
Labor and DeliveryAs with all narcotics, administration of this product to the mother shortly before delivery may result in some degree of respiratory depression in the newborn, especially if higher doses are used.
Nursing MothersAcetaminophen is excreted in breast milk in small amounts, but the significance of its effects on nursing infants is not known. It is not known whether hydrocodone is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from hydrocodone and acetaminophen, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric UseSafety and effectiveness in the pediatric population have not been established.
Geriatric UseClinical studies of hydrocodone bitartrate and acetaminophen tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
HYDROCODONE BITARTRATE AND ACETAMINOPHEN ADVERSE REACTIONS
Central Nervous SystemDrowsiness, mental clouding, lethargy, impairment of mental and physical performance, anxiety, fear, dysphoria, psychic dependence, mood changes.
Gastrointestinal SystemProlonged administration of hydrocodone bitartrate and acetaminophen tablets may produce constipation.
Genitourinary SystemUreteral spasm, spasm of vesical sphincters and urinary retention have been reported with opiates.
Respiratory DepressionHydrocodone bitartrate may produce dose-related respiratory depression by acting directly on the brain stem respiratory center (seeOVERDOSAGE).
Special SensesCases of hearing impairment or permanent loss have been reported predominantly in patients with chronic overdose.
DermatologicalSkin rash, pruritus.
OVERDOSAGEsection.
DRUG ABUSE AND DEPENDENCE
Misuse, Abuse, and Diversion of OpioidsHydrocodone bitartrate and acetaminophen tablets contains hydrocodone, an opioid agonist, and is a Schedule III controlled substance. Hydrocodone bitartrate and acetaminophen tablets, and other opioids used in analgesia can be abused and are subject to criminal diversion.OVERDOSAGE
Signs and Symptoms
HydrocodoneSerious overdose with hydrocodone is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest and death may occur.
AcetaminophenIn acetaminophen overdosage: dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and coagulation defects may also occur.
TreatmentA single or multiple drug overdose with hydrocodone and acetaminophen is a potentially lethal polydrug overdose, and consultation with a regional poison control center is recommended.
DOSAGE & ADMINISTRATION
Product Strength
Usual Adult Dosage as needed for pain
The total 24-hour dosage should not exceed
HOW SUPPLIED
Strength How Supplied Each tablet contains:Description of tablet Hydrocodone Bitartrate Acetaminophen
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Hydrocodone Bitartrate and AcetaminophenHYDROCODONE BITARTRATE TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!