Hydrocodone Bitartrate and Acetaminophen
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
HYDROCODONE BITARTRATE AND ACETAMINOPHEN DESCRIPTION
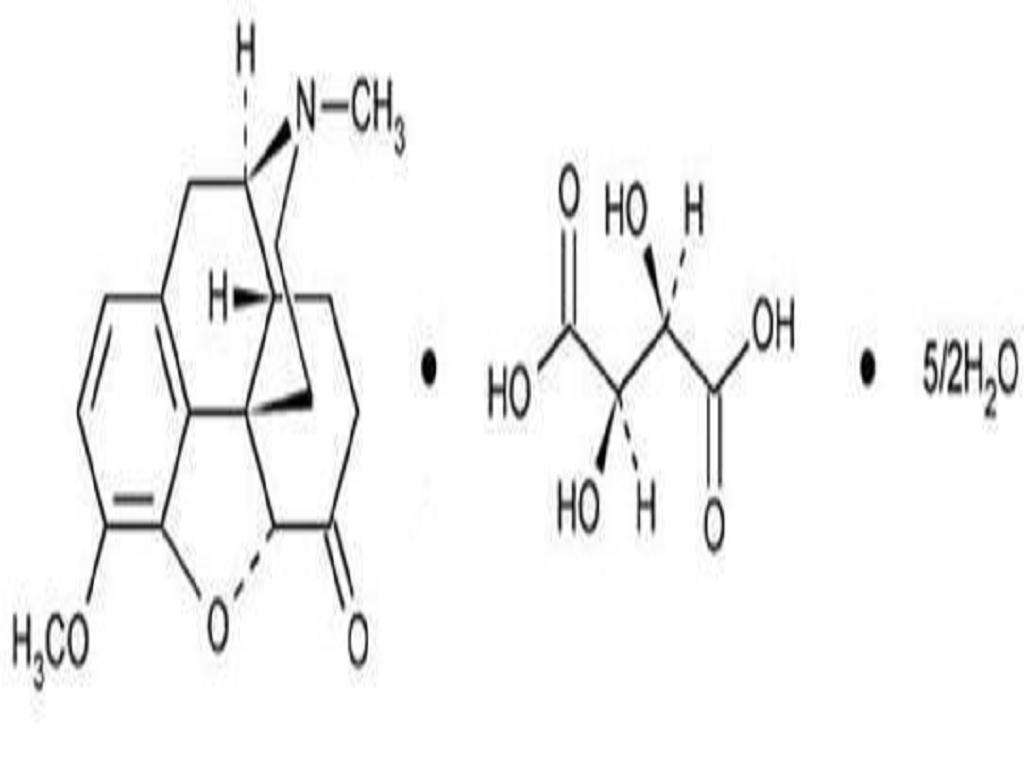
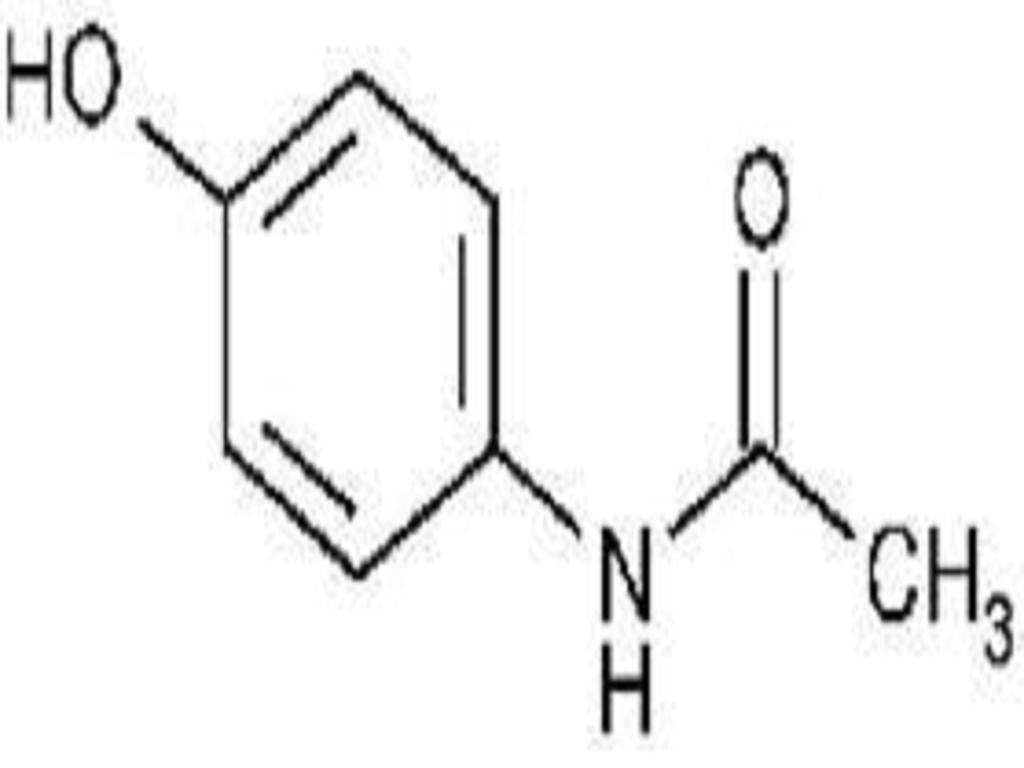
Product StrengthHydrocodone Bitartrate USPAcetaminophen USP
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
OVERDOSAGE
OVERDOSAGE
INDICATIONS & USAGE
HYDROCODONE BITARTRATE AND ACETAMINOPHEN CONTRAINDICATIONS
WARNINGS
Respiratory DepressionHead Injury and Increased Intracranial Pressure
Acute Abdominal Conditions
Misuse, Abuse, and Diversion of Opioids
DRUG ABUSE AND DEPENDENCE
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects:Pregnancy Category CNonteratogenic Effects
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
HYDROCODONE BITARTRATE AND ACETAMINOPHEN ADVERSE REACTIONS
OVERDOSAGE
OVERDOSAGE
DRUG ABUSE AND DEPENDENCE
Misuse, Abuse, and Diversion of OpioidsOVERDOSAGE
Signs and Symptoms:
Treatment
DOSAGE & ADMINISTRATION
Product StrengthUsual Adult Dosage AS NEEDED FOR PAINThe total daily dosage should not exceed
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Hydrocodone Bitartrate and AcetaminophenHydrocodone Bitartrate and Acetaminophen TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!