Hydrocodone Bitartrate and Acetaminophen
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR OWNERS/CAREGIVERS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
HepatotoxicityAcetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen containing product.
HYDROCODONE BITARTRATE AND ACETAMINOPHEN DESCRIPTION
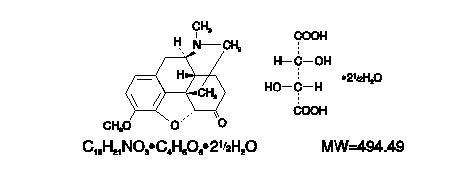
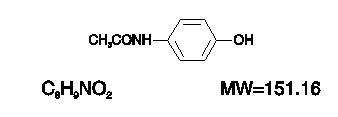
CLINICAL PHARMACOLOGY
Pharmacokinetics:
OVERDOSAGEfor toxicity information.
OVERDOSAGEfor toxicity information.
INDICATIONS & USAGE
HYDROCODONE BITARTRATE AND ACETAMINOPHEN CONTRAINDICATIONS
WARNINGS
HepatotoxicityHypersensitivity/anaphylaxis
Respiratory Depression:At high doses or in sensitive patients, hydrocodone may produce dose-related respiratory depression by acting directly on the brain stem respiratory center. Hydrocodone also affects the center that controls respiratory rhythm, and may produce irregular and periodic breathing.
Head Injury and Increased Intracranial Pressure:The respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries.
Acute Abdominal Conditions:The administration of narcotics may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
PRECAUTIONS
General:Special Risk Patients:As with any narcotic analgesic agent, hydrocodone bitartrate and acetaminophen tablets should be used with caution in elderly or debilitated patients, and those with severe impairment of hepatic or renal function, hypothyroidism, Addison's disease, prostatic hypertrophy or urethral stricture. The usual precautions should be observed and the possibility of respiratory depression should be kept in mind.
Cough Reflex:
INFORMATION FOR OWNERS/CAREGIVERS
LABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
HYDROCODONE BITARTRATE AND ACETAMINOPHEN ADVERSE REACTIONS
Central Nervous System:Drowsiness, mental clouding, lethargy, impairment of mental and physical performance, anxiety, fear, dysphoria, psychic dependence, and mood changes.
Gastrointestinal System:Prolonged administration of hydrocodone bitartrate and acetaminophen tablets may produce constipation.
Genitourinary System:Ureteral spasm, spasm of vesical sphincters, and urinary retention have been reported with opiates.
Respiratory Depression:Hydrocodone bitartrate may produce dose-related respiratory depression by acting directly on the brain stem respiratory center (seeOVERDOSAGE).
Special Senses:Cases of hearing impairment or permanent loss have been reported predominantly in patients with chronic overdose.
Dermatological:Skin rash, pruritus.
OVERDOSAGEsection.
DRUG ABUSE AND DEPENDENCE
Controlled Substance:Hydrocodone Bitartrate and Acetaminophen Tablets are classified as a Schedule III controlled substance.Abuse and Dependence:
OVERDOSAGE
Signs and Symptoms
Hydrocodone:Serious overdose with hydrocodone is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest and death may occur.
Acetaminophen:In acetaminophen overdosage: dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and coagulation defects may also occur. Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
Treatment:A single or multiple drug overdose with hydrocodone and acetaminophen is a potentially lethal polydrug overdose, and consultation with a regional poison control center is recommended. Immediate treatment includes support of cardiorespiratory function and measures to reduce drug absorption. Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. Assisted or controlled ventilation should also be considered.
DOSAGE & ADMINISTRATION
2.5 mg/500 mg
5 mg/500 mg
7.5 mg/325 mg
7.5 mg/500 mg
7.5 mg/650 mg
7.5 mg/750 mg
10 mg/325 mg
10 mg/500 mg
10 mg/650 mg
10 mg/660 mg
10 mg/750 mg
HOW SUPPLIED
WATSON 388on the other side, supplied in bottles of 100.
WATSON 349on the other side, supplied in bottles of 100 and 500.
WATSON 3203on the other side, supplied in bottles of 100.
WATSON 385on the other side, supplied in bottles of 100 and 500.
WATSON 502on the other side, supplied in bottles of 100 and 500.
WATSON 387on the other side, supplied in bottles of 100 and 500.
WATSON 853on the other side, supplied in bottles of 100 and 500.
WATSON 540on the other side, supplied in bottles of 100 and 500.
WATSON 503on the other side, supplied in bottles of 100 and 500.
WATSON 517on the other side, supplied in bottles of 100 and 500.
WATSON 3228on the other side, supplied in bottles of 100.
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Hydrocodone Bitartrate and AcetaminophenHYDROCODONE BITARTRATE TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!