Hydrocortisone
Hydrocortisone Rectal Suspension, USP (Retention)100 mg / 60 mLDISPOSABLE UNIT FOR RECTAL USE ONLY
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDROCORTISONE DESCRIPTION
- CLINICAL PHARMACOLOGY
- HYDROCORTISONE INDICATIONS AND USAGE
- HYDROCORTISONE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- HYDROCORTISONE ADVERSE REACTIONS
- HYDROCORTISONE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PATIENT INSTRUCTIONS
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
HYDROCORTISONE DESCRIPTION
Hydrocortisone is a white to practically white, odorless, crystalline powder, very slightly soluble in water. It has the chemical name Pregn-4-ene-3,20-dione,11,17,21-trihydroxy-,(11β)- and the following structural formula:
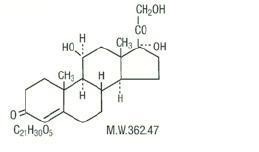
Hydrocortisone Rectal Suspension, USP is a convenient disposable single-dose hydrocortisone enema designed for ease of self-administration.
Each disposable unit (60 mL) contains: Hydrocortisone, 100 mg in an aqueous solution containing carbomer 934P, polysorbate 80, purified water, sodium hydroxide and methylparaben, 0.18% as a preservative.
CLINICAL PHARMACOLOGY
Hydrocortisone is a naturally occurring glucocorticoid (adrenal corticosteroid) similar to its acetate and sodium hemisuccinate derivatives, is partially absorbed following rectal administration. Absorption studies in ulcerative colitis patients have shown up to 50% absorption of hydrocortisone administered as Hydrocortisone Rectal Suspension, USP and up to 30% of hydrocortisone acetate administered in an identical vehicle.
Hydrocortisone Rectal Suspension, USP provides the potent anti-inflammatory effect of hydrocortisone. Because this drug is absorbed from the colon, it acts both topically and systemically. Although rectal hydrocortisone, used as recommended for Hydrocortisone Rectal Suspension, USP, has a low incidence of reported adverse reactions, prolonged use presumably may cause systemic reactions associated with oral dosage forms.
HYDROCORTISONE INDICATIONS AND USAGE
Hydrocortisone Rectal Suspension, USP is indicated as adjunctive therapy in the treatment of ulcerative colitis, especially distal forms, including ulcerative proctitis, ulcerative proctosigmoiditis, and left-sided ulcerative colitis. It has proved useful also in some cases involving the transverse and ascending colons.
HYDROCORTISONE CONTRAINDICATIONS
Systemic fungal infections; and ileocolostomy during the immediate or early post-operative period.
WARNINGS
In severe ulcerative colitis, it is hazardous to delay needed surgery while awaiting response to medical treatment.
Damage to the rectal wall can result from careless or improper insertion of an enema tip.
In patients on corticosteroid therapy subjected to unusual stress, increased dosage of rapidly acting corticosteroids before, during, and after the stressful situation is indicated.
Corticosteroids may mask some signs of infection, and new infections may appear during their use. There may be decreased resistance and inability to localize infection when corticosteroids are used.
Prolonged use of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to fungi or viruses.
Usage in Pregnancy:
Since adequate human reproduction studies have not been done with corticosteroids, the use of these drugs in pregnancy, nursing mothers or women of child-bearing potential requires that the possible benefits of the drug be weighed against the potential hazards to the mother and embryo or fetus. Neonates born of mothers who have received substantial doses of corticosteroid during pregnancy should be carefully observed for signs of hypoadrenalism.
Average and large doses of hydrocortisone or cortisone can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when used in large doses. Dietary salt restriction and potassium supplementation may be necessary. All corticosteroids increase calcium excretion.
While on corticosteroid therapy, patients should not be vaccinated against smallpox. Other immunization procedures should not be undertaken in patients who are on corticosteroids, especially on high dose, because of possible hazards of neurological complications and a lack of antibody response.
Persons who are on drugs which suppress the immune system are more susceptible to infections than healthy individuals. Chicken pox and measles, for example, can have a more serious or even fatal course in non-immune pediatric patients or adults on corticosteroids. In such pediatric patients or adults who have not had these diseases, particular care should be taken to avoid exposure. How the dose, route and duration of corticosteroid administration affects the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chicken pox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chicken pox develops, treatment with antiviral agents may be considered.
If corticosteroids are indicated in patients with latent tuberculosis or tuberculin reactivity, close observation is necessary as reactivation of the disease may occur. During prolonged corticosteroid therapy, these patients should receive chemoprophylaxis.
PRECAUTIONS
General:
Hydrocortisone Rectal Suspension, USP should be used with caution where there is a probability of impending perforation, abscess or other pyogenic infection; fresh intestinal anastomoses; obstruction; or extensive fistulas and sinus tracts. Use with caution in presence of active or latent peptic ulcer; diverticulitis; renal insufficiency; hypertension; osteoporosis; and myasthenia gravis.
Steroid therapy might impair prognosis in surgery by increasing the hazard of infection. If infection is suspected, appropriate antibiotic therapy must be administered, usually in larger than ordinary doses.
Drug-induced secondary adrenocortical insufficiency may occur with prolonged Hydrocortisone Rectal Suspension, USP therapy. This is minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. Since mineralocorticoid secretion may be impaired, salt and/or a mineralocorticoid should be administered concurrently.
There is an enhanced effect of corticosteroids on patients with hypothyroidism and in those with cirrhosis.
Corticosteroid should be used cautiously in patients with ocular herpes simplex because of possible corneal perforation.
The lowest possible dose of corticosteroid should be used to control the conditions under treatment, and when reduction in dosage is possible, the reduction should be gradual.
Psychic derangement may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes, and severe depression, to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids.
Aspirin should be used cautiously in conjunction with corticosteroids in hypoprothrombinemia.
Pediatric Use:
Safety and effectiveness in pediatric patients have not been established.
Growth and development of pediatric patients on prolonged corticosteroid therapy should be carefully observed.
Information for Patients:
Persons who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chicken pox or measles. Patients should also be advised that if they are exposed, medical advice should be sought without delay.
HYDROCORTISONE ADVERSE REACTIONS
Local pain or burning and rectal bleeding attributed to Hydrocortisone Rectal Suspension, USP have been reported rarely. Apparent exacerbations or sensitivity reactions also occur rarely. The following adverse reactions should be kept in mind whenever corticosteroids are given by rectal administration.
Fluid and Electrolyte Disturbances: Sodium retention; fluid retention; congestive heart failure in susceptible patients; potassium loss; hypokalemic alkalosis; hypertension. Musculoskeletal: Muscle weakness; steroid myopathy; loss of muscle mass; osteoporosis; vertebral compression fractures; asceptic necrosis of femoral and humeral heads; pathologic fracture of long bones. Gastrointestinal: Peptic ulcer with possible perforation and hemorrhage; pancreatitis; abdominal distention; ulcerative esophagitis. Dermatologic: Impaired wound healing; thin fragile skin; petechiae and ecchymoses; facial erythema; increased sweating; may suppress reactions to skin tests. Neurological: Convulsions; increased intracranial pressure with papilledema (pseudo-tumor cerebri) usually after treatment; vertigo; headache. Endocrine: Menstrual irregularities; development of Cushingoid state; suppression of growth in pediatric patients; secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma, surgery or illness, decreased carbohydrate tolerance; manifestations of latent diabetes requirements for insulin or oral hypoglycemic agents in diabetics. Ophthalmic: Posterior subcapsular cataracts; increased intraocular pressure; glaucoma; exophthalmos. Metabolic: Negative nitrogen balance due to protein catabolism.
HYDROCORTISONE DOSAGE AND ADMINISTRATION
The use of Hydrocortisone Rectal Suspension, USP hydrocortisone retention enema is predicated upon the concomitant use of modern supportive measures such as rational dietary control, sedatives, antidiarrheal agents, antibacterial therapy, blood replacement if necessary, etc.
The usual course of therapy is one Hydrocortisone Rectal Suspension, USP nightly for 21 days, or until the patient comes into remission both clinically and proctologically. Clinical symptoms usually subside promptly within 3 to 5 days. Improvement in the appearance of the mucosa, as seen by sigmoidoscopic examination, may lag somewhat behind clinical improvement. Difficult cases may require as long as 2 or 3 months of Hydrocortisone Rectal Suspension, USP treatment. Where the course of therapy extends beyond 21 days, Hydrocortisone Rectal Suspension, USP should be discontinued gradually by reducing administration to every other night for 2 or 3 weeks.
If clinical or proctologic improvement fails to occur within 2 or 3 weeks after starting Hydrocortisone Rectal Suspension, USP, discontinue its use.
Symptomatic improvement, evidenced by decreased diarrhea and bleeding; weight gain; improved appetite; lessened fever; and decrease in leukocytosis, may be misleading and should not be used as the sole criterion in judging efficacy. Sigmoidoscopic examination and X-ray visualization are essential for adequate monitoring of ulcerative colitis. Biopsy is useful for differential diagnosis.
Patient instructions for administering Hydrocortisone Rectal Suspension, USP are enclosed in each box. It is recommended that the patient lie on their left side during administration and for 30 minutes thereafter, so that the fluid will distribute throughout the left colon. Every effort should be made to retain the enema for at least an hour and preferably, all night. This may be facilitated by prior sedation and/or antidiarrheal medication, especially early in therapy when the urge to evacuate is great.
HOW SUPPLIED
Hydrocortisone Rectal Suspension, USP (Retention)100 mg retention enema, is supplied as disposable single-dose bottles with lubricated rectal applicator tips, in boxes of seven x 60 mL (NDC 42769-1380-7) and boxes of one x 60 mL (NDC 42769-1380-1).
Store at controlled room temperature 20° - 25°C (68° - 77°F). [See USP Controlled Room Temperature.]
PATIENT INSTRUCTIONS
Rx ONLY
How to use the retention enema:
Best results are achieved if the bowel is emptied immediately before the enema is given.
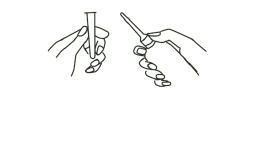
1. Preparing the Medication for Administration
a Shake the bottle well to make sure that the suspension is homogeneous.
b Remove the protective sheath from the applicator tip. Hold the bottle at the neck so as not to cause any of the medication to be discharged.
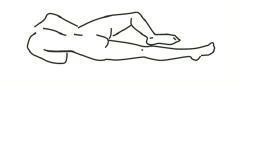
2. Assuming the Correct Body Position
a Best results are obtained by lying on left side with the left leg extended and the right leg flexed forward for balance.
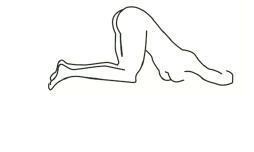
b An alternative to lying on the left side is the “knee-chest” position as shown here.
3. Administering the Retention Enema
a Gently insert the lubricated applicator tip into the rectum, pointed slightly toward the navel (umbilicus).
b Grasp the bottle firmly, then tilt slightly so that the nozzle is aimed toward the back, and squeeze slowly to instill the medication. Steady hand pressure will discharge most of the solution. After administering, withdraw and discard the used unit.
c Remain in position for at least 30 minutes to allow thorough distribution of the medication internally. Retain the enema all night, if possible.
BayPharma™
Manufactured for
BayPharma™, Inc.
Baltimore, MD 21244
571-138051-0
Rev 06/08
Package/Label Principal Display Panel
NDC 42769-1380-1
UNIT DOSE 1 x 60 mL
THIS END UP
Hydrocortisone Rectal Suspension, USP (Retention) 100 mg/60 mL
USUAL DOSE : One 60 mL enema daily before retiring.
See enclosed directions for use. See package insert for additional prescribing information.
Each disposable unit (60 mL) contains: Hydrocortisone, 100 mg in an
aqueous solution containing carbomer 934P, polysorbate 80, purified
water, sodium hydroxide and methylparaben, 0.18% as a preservative.
FOR RECTAL USE ONLY.
SHAKE WELL BEFORE USE.
Store at controlled room temperature, 20°-25°C (68°-77°F)
[See USP Controlled Temperature].
Rx only
BayPharma, Inc., Baltimore, MD 21244

HydrocortisoneHydrocortisone ENEMA
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||