Hydroxocobalamin
HYDROXOCOBALAMIN INJECTION USP Rx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDROXOCOBALAMIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- HYDROXOCOBALAMIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- HYDROXOCOBALAMIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
HYDROXOCOBALAMIN DESCRIPTION
Hydroxocobalamin injection is a sterile solution of hydroxocobalamin for intramuscular administration.
Each mL contains: Hydroxocobalamin Acetate equivalent to 1000 mcg Hydroxocobalamin, Sodium Acetate Anhydrous 0.2 mg, Glacial Acetic Acid 0.442 mg, Sodium Chloride 8.2 mg, with Methylparaben 1.5 mg and Propylparaben 0.2 mg as preservatives, in Water for Injection q.s. Additional Glacial Acetic Acid and/or Sodium Acetate may have been used to adjust pH. pH range is 3.5 to 5.0.
Hydroxocobalamin appears as dark red orthorhombic needles or as an amorphous or crystalline red powder. It is very hygroscopic in the anhydrous form, and moderately soluble in water. It has a molecular weight of 1346.37. The vitamin B12 coenzymes are very unstable in light. Hydroxocobalamin shares the cobalamin molecular structure with cyanocobalamin.
The chemical name is α-(5,6-dimethylbenzimidazoly) hydroxocobamide. The empirical formula is C62H89CoN13O15P and its structural formula is:
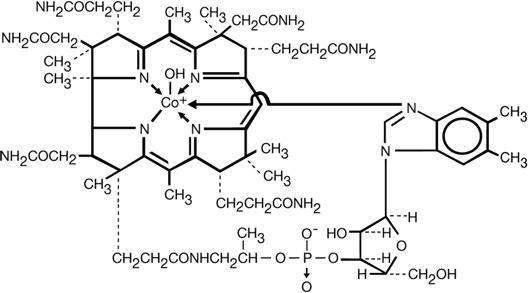
The cobalt content is 4.34%.
CLINICAL PHARMACOLOGY
Vitamin B12 is essential to growth, cell reproduction, hematopoiesis, nucleoprotein and myelin synthesis.
Fifty percent of the administered dose of hydroxocobalamin disappears from the injection site in 2.5 hours. Hydroxocobalamin is bound to plasma proteins and stored in the liver. It is excreted in the bile and undergoes some enterohepatic recycling. Within 72 hours after injection of 500 to 1000 mcg of hydroxocobalamin, 16 to 66 percent of the injected dose may appear in the urine. The major portion is excreted within the first 24 hours.
INDICATIONS & USAGE
-
Pernicious anemia, both uncomplicated and accompanied by nervous system involvement.
-
Dietary deficiency of Vitamin B12, occurring in strict vegetarians and in their breast-fed infants. (Isolated vitamin B12 deficiency is very rare).
-
Malabsorption of vitamin B12, resulting from structural or functional damage to the stomach, where intrinsic factor is secreted or to the ileum, where intrinsic factor facilitates vitamin B12 absorption. These conditions include tropical sprue, and nontropical sprue (idiopathic steatorrhea, gluten-induced enteropathy). Folate deficiency in these patients is usually more severe than vitamin B12 deficiency.
-
Inadequate secretion of intrinsic factor, resulting from lesions that destroy the gastric mucosa (ingestion of corrosives, extensive neoplasia), and a number of conditions associated with a variable degree of gastric atrophy (such as multiple sclerosis, certain endocrine disorders, iron deficiency, and subtotal gastrectomy). Total gastrectomy always produces vitamin B12 deficiency.
Structural lesions leading to vitamin B12 deficiency include regional ileitis, ileal resections, malignancies, etc.
-
Competition for Vitamin B12 by intestinal parasites or bacteria.
The fish tapeworm (Diphyilobothrium latum) absorbs huge quantities of vitamin B12 and infested patients often have associated gastric atrophy. The blind-loop syndrome may produce deficiency of Vitamin B12 or folate.
-
Inadequate utilization of vitamin B12. This may occur if antimetabolites for the vitamin are employed in the treatment of neoplasia.
-
For the Schilling Test.
HYDROXOCOBALAMIN CONTRAINDICATIONS
Hypersensitivity to any component of this medication.
WARNINGS
Avoid the intravenous route.
Folic acid is not a substitute for vitamin B12 although it may improve vitamin B12 deficient megaloblastic anemia. Exclusive use of folic acid in treating vitamin B12 deficient megaloblastic anemia could result in progressive and irreversible neurologic damage.
Blunted or impeded therapeutic response to vitamin B12 may be due to such conditions as infection, uremia, drugs having bone marrow suppressant properties such as chloramphenicol, and concurrent iron or folic acid deficiency.
PRECAUTIONS
The validity of diagnostic vitamin B12 or folic acid blood assays could be compromised by medications, and this should be considered before relying on such tests for therapy.
Vitamin B12 is not a substitute for folic acid and since it might improve folic acid deficient megaloblastic anemia, indiscriminate use of vitamin B12 could mask the true diagnosis.
Hypokalemia and thrombocytosis could occur upon conversion of severe megaloblastic to normal erythropoiesis with B12 therapy. Therefore, serum potassium levels and the platelet count should be monitored carefully during therapy.
Vitamin B12 deficiency may suppress the signs of polycythemia vera. Treatment with vitamin B12 may unmask this condition.
Studies of carcinogenicity, mutagenesis, or impairment of fertility have not been performed with hydroxocobalamin.
Teratogenic Effects: Pregnancy Category C: Animal reproduction studies have not been conducted with hydroxocobalamin. It is also not known whether hydroxocobalamin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Hydroxocobalamin should be given to a pregnant woman only if clearly needed.
HYDROXOCOBALAMIN ADVERSE REACTIONS
Mild transient diarrhea, itching, transitory exanthema, feeling of swelling of entire body, and anaphylaxis.
A few patients may experience pain after injection of hydroxocobalamin.
OVERDOSAGE
The intravenous LD50 of hydroxocobalamin in mice is greater than 50 mL/kg.
DOSAGE & ADMINISTRATION
Protect from light.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Hydroxocobalamin injection should be given only intramuscularly.
In patients with Addisonian Pernicious Anemia, parenteral therapy with vitamin B12 is the recommended method of treatment and will be required for the remainder of the patient’s life. Oral therapy is not dependable. In other patients with vitamin B12 deficiency, the duration of therapy and route of administration will depend upon the cause and whether or not it is reversible.
Confirmatory diagnostic studies should be performed prior to initiating therapy, if possible, and the patient should be followed with appropriate studies to demonstrate hematologic improvement (Hgb, hematocrit, RBC, reticulocyte count). A diagnostic trial utilizing physiologic doses of vitamin B12 (1 mcg daily) and observing daily reticulocyte counts after establishing a baseline may also be performed. The observation of reticulocytosis which usually occurs between the third and tenth day of therapy confirms the diagnosis of vitamin B12 deficiency.
In seriously ill patients it may be advisable to administer both vitamin B12 and folic acid while awaiting the results of distinguishing laboratory studies. It is not necessary to withhold vitamin B12 therapy until the precise cause of B12 deficiency is established since absorption studies can be performed at any time.
Serum potassium should be closely observed the first 48 hours and potassium should be administered if necessary.
Thirty mcg daily for 5 to 10 days followed by 100 to 200 mcg monthly injected intramuscularly. If the patient is critically ill, or has neurologic disease, an infectious disease or hyperthyroidism, considerably higher doses may be indicated. However, current data indicate that the optimum obtainable neurologic response may be expected with a dosage of vitamin B12 sufficient to produce good hematologic response. Children may be given a total of 1 to 5 mg over a period of 2 or more weeks in doses of 100 mcg, then 30 to 50 mcg every 4 weeks for maintenance.
Patients who have normal intestinal absorption may be treated with an oral therapeutic multivitamin preparation, containing 15 mcg vitamin B12 daily.
The flushing dose is 1000 mcg.
HOW SUPPLIED
Hydroxocobalamin Injection USP, 1000 mcg/mL is available in a 30 mL multiple dose vial, individually boxed.
Store at 20°-25°C (68°-77°F). [See USP controlled room temperature.] PROTECT FROM LIGHT.
Literature revised: July 2010
Product No.: 0208-30
Manufactured by:
Hikma Farmaceutica (Portugal)
S.A. 2705-906 Terrugem SNT, Portugal
Distributed by:
Watson Pharma, Inc.
Corona, CA 92880 USA PIN229-WAT/1
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
DRUG: Hydroxocobalamin
GENERIC: Hydroxocobalamin
DOSAGE: INJECTION, SOLUTION
ADMINSTRATION: INTRAMUSCULAR
NDC: 52125-799-01
ACTIVE INGREDIENT(S):
- HYDROXOCOBALAMIN ACETATE 1000ug in 1mL
INACTIVE INGREDIENT(S):
- METHYLPARABEN
- PROPYLPARABEN
- SODIUM ACETATE ANHYDROUS
- ACETIC ACID
- SODIUM CHLORIDE
- WATER
PACKAGING: 30 mL in 1 VIAL, MULTI-DOSE

HydroxocobalaminHydroxocobalamin INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||