HydrOXYzine Hydrochloride
NorthStar RxLLC
Piramal Enterprises Limited
Rx Only
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDROXYZINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- HYDROXYZINE HYDROCHLORIDE INDICATIONS AND USAGE
- HYDROXYZINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- HYDROXYZINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- HYDROXYZINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Principal Display Panel - HydrOXYzine Hydrochloride Tablets USP, 10 mg - 100's Pack
- Principal Display Panel - HydrOXYzine Hydrochloride Tablets USP, 25 mg - 100's Pack
- Principal Display Panel - HydrOXYzine Hydrochloride Tablets USP ,50 mg - 100's Pack
FULL PRESCRIBING INFORMATION
HYDROXYZINE HYDROCHLORIDE DESCRIPTION
HydrOXYzine hydrochloride has the chemical name of (±)-2-[2-[4-(p-Chloro-α-phenylbenzyl)-1-piperazinyl] ethoxy]ethanol dihydrochloride.
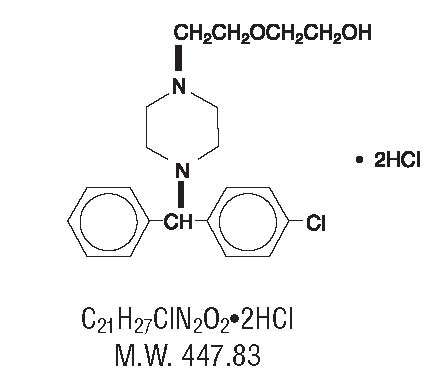
HydrOXYzine hydrochloride occurs as a white, odorless powder which is very soluble in water; soluble in chloroform; slightly soluble in acetone; practically insoluble in ether.
Each tablet for oral administration contains 10 mg, 25 mg or 50 mg hydrOXYzine hydrochloride USP.
Inactive ingredients for 10 mg include anhydrous lactose, colloidal silicon dioxide, crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch and titanium dioxide.
Inactive ingredients for 25 mg include anhydrous lactose, colloidal silicon dioxide, crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch, sodium starch glycolate and titanium dioxide.
Inactive ingredients for 50 mg include anhydrous lactose, colloidal silicon dioxide, crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch, sodium starch glycolate and titanium dioxide.
CLINICAL PHARMACOLOGY
HydrOXYzine hydrochloride is unrelated chemically to the phenothiazines, reserpine, meprobamate or the benzodiazepines. HydrOXYzine is not a cortical depressant, but its action may be due to a suppression of activity in certain key regions of the subcortical area of the central nervous system.
Primary skeletal muscle relaxation has been demonstrated experimentally. Bronchodilator activity, and antihistaminic and analgesic effects have been demonstrated experimentally and confirmed clinically. An antiemetic effect, both by the apomorphine test and the veriloid test, has been demonstrated.
Pharmacological and clinical studies indicate that hydrOXYzine in therapeutic dosage does not increase gastric secretion or acidity and in most cases has mild antisecretory activity.
HydrOXYzine is rapidly absorbed from the gastrointestinal tract and hydrOXYzine's clinical effects are usually noted within 15 to 30 minutes after oral administration.
HYDROXYZINE HYDROCHLORIDE INDICATIONS AND USAGE
For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested.
Useful in the management of pruritus due to allergic conditions such as chronic urticaria and atopic and contact dermatoses and in histamine-mediated pruritus.
As a sedative when used as a premedication and following general anesthesia, hydrOXYzine may potentiate meperidine and barbiturates, so their use in pre-anesthetic adjunctive therapy should be modified on an individual basis. Atropine and other belladonna alkaloids are not affected by the drug. HydrOXYzine is not known to interfere with the action of digitalis in any way and it may be used concurrently with this agent.
The effectiveness of hydrOXYzine as an antianxiety agent for long term use, that is more than 4 months, has not been assessed by systematic clinical studies. The physician should reassess periodically the usefulness of the drug for the individual patient.
HYDROXYZINE HYDROCHLORIDE CONTRAINDICATIONS
Oral hydrOXYzine hydrochloride is contraindicated in patients with known hypersensitivity to hydrOXYzine hydrochloride products, and in patients with known hypersensitivity to cetirizine hydrochloride or levocetirizine hydrochloride.
HydrOXYzine, when administered to the pregnant mouse, rat, and rabbit induced fetal abnormalities in the rat and mouse at doses substantially above the human therapeutic range. Clinical data in human beings are inadequate to establish safety in early pregnancy. Until such data are available, hydrOXYzine is contraindicated in early pregnancy.
HydrOXYzine is contraindicated for patients who have shown a previous hypersensitivity to any component of this medication.
WARNINGS
Nursing Mothers
It is not known whether this drug is excreted in human milk. Since many drugs are so excreted, hydrOXYzine should not be given to nursing mothers.
PRECAUTIONS
THE POTENTIATING ACTION OF HYDROXYZINE MUST BE CONSIDERED WHEN THE DRUG IS USED IN CONJUNCTION WITH CENTRAL NERVOUS SYSTEM DEPRESSANTS SUCH AS NARCOTICS, NON-NARCOTIC ANALGESICS AND BARBITURATES. Therefore, when central nervous system depressants are administered concomitantly with hydrOXYzine their dosage should be reduced.
Since drowsiness may occur with use of this drug, patients should be warned of this possibility and cautioned against driving a car or operating dangerous machinery while taking hydrOXYzine. Patients should also be advised against the simultaneous use of other CNS depressant drugs, and cautioned that the effects of alcohol may be increased.
Geriatric Use
A determination has not been made whether controlled clinical studies of hydrOXYzine included sufficient numbers of subjects aged 65 and over to define a difference in response from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
The extent of renal excretion of hydrOXYzine has not been determined. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selections.
Sedating drugs may cause confusion and over sedation in the elderly; elderly patients generally should be started on low doses of hydrOXYzine and observed closely.
HYDROXYZINE HYDROCHLORIDE ADVERSE REACTIONS
Side effects reported with the administration of hydrOXYzine hydrochloride are usually mild and transitory in nature.
Anticholinergic: Dry mouth.
Central Nervous System: Drowsiness is usually transitory and may disappear in a few days of continued therapy or upon reduction of dose. Involuntary motor activity including rare instances of tremor and convulsions have been reported, usually with doses considerably higher than those recommended. Clinically significant respiratory depression has not been reported at recommended doses.
In post-marketing experience, the following additional undesirable effects have been reported:
Body as a Whole: allergic reaction.
Nervous System : headache.
Psychiatric: hallucination.
Skin and Appendages: Oral hydrOXYzine hydrochloride is associated with fixed eruptions in postmarketing reports. Pruritus, rash, urticaria.
OVERDOSAGE
The most common manifestation of hydrOXYzine overdosage is hypersedation.Other reported signs and symptoms were convulsions, stupor, nausea and vomiting.As in the management of overdosage with any drug, it should be borne in mind that multiple agents may have been taken.
If vomiting has not occurred spontaneously, it should be induced. Immediate gastric lavage is also recommended. General supportive care, including frequent monitoring of the vital signs and close observation of the patient, is indicated. Hypotension, though unlikely, may be controlled with intravenous fluids and levarterenol or metaraminol. Do not use epinephrine as hydrOXYzine counteracts its pressor action.
There is no specific antidote. It is doubtful that hemodialysis would be of any value in the treatment of overdosage with hydrOXYzine. However, if other agents such as barbiturates have been ingested concomitantly, hemodialysis may be indicated. There is no practical method to quantitate hydrOXYzine in body fluids or tissue after its ingestion or administration.
HYDROXYZINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested: Adults, 50 to 100 mg q.i.d.; children under 6 years, 50 mg daily in divided doses; children over 6 years, 50 to 100 mg daily in divided doses.
For use in the management of pruritus due to allergic conditions such as chronic urticaria and atopic and contact dermatoses and in histamine-mediated pruritus: adults, 25 mg t.i.d. or q.i.d.; children under 6 years, 50 mg daily in divided doses; children over 6 years, 50 to 100 mg daily in divided doses.
As a sedative when used as a premedication and following general anesthesia: 50 to 100 mg for adults and 0.6 mg/kg of body weight in children.
When treatment is initiated by the intramuscular route of administration, subsequent doses may be administered orally.
As with all potent medication, the dosage should be adjusted according to the patient's response to therapy.
HOW SUPPLIED
HydrOXYzine Hydrochloride Tablets, USP are supplied as:
10 mg - White, biconvex, circular, coated tablets with '112' debossed on one face and 'N' on the other face.
Bottles of 30 tablets NDC 16714-081-01
Bottles of 60 tablets NDC 16714-081-02
Bottles of 90 tablets NDC 16714-081-03
Bottles of 100 tablets NDC 16714-081-04
Bottles of 500 tablets NDC 16714-081-05
Bottles of 1000 tablets NDC 16714-081-06
Bottles of 30 tablets NDC 16714-081-07
Bottles of 60 tablets NDC 16714-081-08
Bottles of 90 tablets NDC 16714-081-09
Bottles of 100 tablets NDC 16714-081-10
Bottles of 500 tablets NDC 16714-081-11
Bottles of 1000 tablets NDC 16714-081-12
25 mg- White, biconvex, circular, coated tablets with 'N004' debossed on one face and plain on the other face.
Bottles of 30 tablets NDC 16714-082-01
Bottles of 60 tablets NDC 16714-082-02
Bottles of 90 tablets NDC 16714-082-03
Bottles of 100 tablets NDC 16714-082-04
Bottles of 500 tablets NDC 16714-082-05
Bottles of 1000 tablets NDC 16714-082-06
Bottles of 30 tablets NDC 16714-082-07
Bottles of 60 tablets NDC 16714-082-08
Bottles of 90 tablets NDC 16714-082-09
Bottles of 100 tablets NDC 16714-082-10
Bottles of 500 tablets NDC 16714-082-11
Bottles of 1000 tablets NDC 16714-082-12
50 mg- White, biconvex, circular, coated tablets with 'N006' debossed on one face and plain on the other face.
Bottles of 30 tablets NDC 16714-083-01
Bottles of 60 tablets NDC 16714-083-02
Bottles of 90 tablets NDC 16714-083-03
Bottles of 100 tablets NDC 16714-083-04
Bottles of 500 tablets NDC 16714-083-05
Bottles of 1000 tablets NDC 16714-083-06
Bottles of 30 tablets NDC 16714-083-07
Bottles of 60 tablets NDC 16714-083-08
Bottles of 90 tablets NDC 16714-083-09
Bottles of 100 tablets NDC 16714-083-10
Bottles of 500 tablets NDC 16714-083-11
Bottles of 1000 tablets NDC 16714-083-12
Dispense in a tight container as defined in the USP, with a child-resistant closure (as required).
Store at 20° to 25° C (68° to 77° F). [See USP Controlled Room Temperature].
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured for:
Northstar Rx LLC
Memphis, TN 38141
Toll Free : 1-800-206-7821
Manufactured by:
Piramal Enterprises Limited,
Plot No. 67-70, Sector-2,
Pithampur 454 775, Dist.: Dhar,
Madhya Pradesh, INDIA.
April 2014
Principal Display Panel - HydrOXYzine Hydrochloride Tablets USP, 10 mg - 100's Pack
LABELS OF RXPAK

Rx only
NDC 16714-081-04 Rev. 09/12
HydrOXYzine Hydrochloride Tablets, USP 10 mg
100 Tablets
NORTHSTAR
Each tablet contains 10 mg of hydrOXYzine hydrochloride,USP.
Usual dosage: See package outsert for full prescribing information.
Dispense in a tight container as defined in the USP, with a child-resistant closure (as required).
Storage: Store at 20°C to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured for: Northstar Rx LLC
Memphis, TN38141
Toll Free: 1-800-206-7821
Manufactured by: Piramal Enterprises Limited
Pithampur, Madhya Pradesh
454775, INDIA
Mfg Lic. No.: 25/10/92
Bar code
Batch No.:
Exp. Date:
LABELS OF PITHAMPUR

Rx only
NDC 16714-081-10
HydrOXYzine Hydrochloride Tablets, USP 10 mg
100 Tablets
NORTHSTAR
Each tablet contains 10 mg of hydrOXYzine hydrochloride,USP.
Usual dosage: See package outsert for full prescribing information.
Dispense in a tight container as defined in the USP, with a child-resistant closure (as required).
Storage: Store at 20°C to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured for: Northstar Rx LLC
Memphis, TN38141. Toll Free: 1-800-206-7821
Manufactured by: Piramal Enterprises Limited, Plot No. 67 - 70,
Sector - 2, Pithampur 454775, Dist. Dhar, Madhya Pradesh, INDIA.
Mfg Lic. No.: 25/10/92 20614994 EM 14793/a Rev. 05/13
Bar code
Principal Display Panel - HydrOXYzine Hydrochloride Tablets USP, 25 mg - 100's Pack
LABELS OF RXPAK

Rx only
NDC 16714-082-04 Rev.09/12
HydrOXYzine Hydrochloride Tablets, USP 25 mg
100 Tablets
NORTHSTAR
Each tablet contains 25 mg of hydrOXYzine hydrochloride,USP.
Usual dosage: See package outsert for full prescribing information.
Dispense in a tight container as defined in the USP, with a child-resistant closure (as required).
Storage: Store at 20°C to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured for: Northstar Rx LLC
Memphis, TN38141
Toll-free: 1-800-206-7821
Manufactured by: Piramal Enterprises Limited
Pithampur, Madhya Pradesh
454775, INDIA
Mfg Lic. No.: 25/10/92
Bar Code
Batch No.:
Exp. Date:
LABELS OF PITHAMPUR

Rx only
NDC 16714-082-10
HydrOXYzine Hydrochloride Tablets, USP 25 mg
100 Tablets
NORTHSTAR
Each tablet contains 25 mg of hydrOXYzine hydrochloride,USP.
Usual dosage: See package outsert for full prescribing information.
Dispense in a tight container as defined in the USP, with a child-resistant closure (as required).
Storage: Store at 20°C to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured for: Northstar Rx LLC
Memphis, TN38141. Toll-free: 1-800-206-7821
Manufactured by: Piramal Enterprises Limited, Plot No. 67 - 70,
Sector - 2, Pithampur 454775, Dist. Dhar, Madhya Pradesh, INDIA.
Mfg Lic. No.: 25/10/92 20615001 EM 14795/a Rev. 05/13
Bar Code
Principal Display Panel - HydrOXYzine Hydrochloride Tablets USP ,50 mg - 100's Pack
LABELS OF RXPAK

Rx only
NDC 16714-083-04 Rev. 09/12
HydrOXYzine Hydrochloride Tablets, USP 50 mg
100 Tablets
NORTHSTAR
Each tablet contains 50 mg of hydrOXYzine hydrochloride,USP.
Usual dosage: See package outsert for full prescribing information.
Dispense in a tight container as defined in the USP, with a child-resistant closure (as required).
Storage: Store at 20°C to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured for: Northstar Rx LLC
Memphis, TN38141
Toll-free : 1-800-206-7821
Manufactured by: Piramal Enterprises Limited
Pithampur, Madhya Pradesh
454775, INDIA
Mfg Lic. No.: 25/10/92
Bar Code
Batch No.:
Exp. Date:
LABELS OF PITHAMPUR

Rx only
NDC 16714-083-10
HydrOXYzine Hydrochloride Tablets, USP 50 mg
100 Tablets
NORTHSTAR
Each tablet contains 50 mg of hydrOXYzine hydrochloride,USP.
Usual dosage: See package outsert for full prescribing information.
Dispense in a tight container as defined in the USP, with a child-resistant closure (as required).
Storage: Store at 20°C to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured for: Northstar Rx LLC
Memphis, TN38141. Toll-free : 1-800-206-7821
Manufactured by: Piramal Enterprises Limited, Plot No. 67 - 70,
Sector - 2, Pithampur 454775, Dist. Dhar, Madhya Pradesh, INDIA.
Mfg Lic. No.: 25/10/92
Bar Code
HydrOXYzine HydrochlorideHydrOXYzine Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
HydrOXYzine HydrochlorideHydrOXYzine Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
HydrOXYzine HydrochlorideHydrOXYzine Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||