Hydroxyzine
General Injectables & Vaccines, Inc
Hydroxyzine HCL 25 mg/mL Injection, USP 1mL Single Dose Vial
FULL PRESCRIBING INFORMATION: CONTENTS*
- Description
- Clinical Pharmacology
- Hydroxyzine Indications and Usage
- Contraindications
- Precautions
- Side Effects
- Dosage and Administration
- How Supplied
- Sample Package Label
FULL PRESCRIBING INFORMATION
Description
Rx Only
Hydroxyzine hydrochloride has the chemical name of (±)-2-[2-[4-(p-Chloro-a-phenylbenzyl)-1-piperazinyl]ethoxy]ethanol dihydrochloride and occurs as a white, odorless powder which is very soluble in water.
It has the following structural formula:
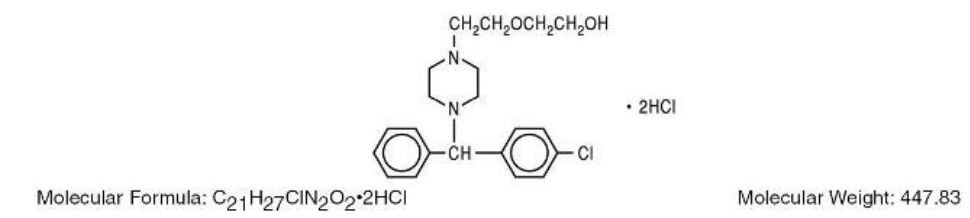
Hydroxyzine Hydrochloride Injection, USP is a sterile aqueous solution intended for intramuscular administration. Each mL contains: Hydroxyzine HCl 25 mg or 50 mg, Benzyl Alcohol 0.9%, and Water for Injection q.s. pH adjusted with Sodium Hydroxide and/or Hydrochloric Acid.
Clinical Pharmacology
Hydroxyzine hydrochloride is unrelated chemically to phenothiazine, reserpine, and meprobamate. Hydroxyzine has demonstrated its clinical effectiveness in the chemotherapeutic aspect of the total management of neuroses and emotional disturbances manifested by anxiety, tension, agitation, apprehension or confusion. Hydroxyzine has been shown clinically to be a rapid-acting true ataraxic with a wide margin of safety. It induces a calming effect in anxious, tense, psychoneurotic adults and also in anxious, hyperkinetic children without impairing mental alertness. It is not a cortical depressant, but its action may be due to a suppression of activity in certain key regions of the subcortical area of the central nervous system.
Primary skeletal muscle relaxation has been demonstrated experimentally.
Hydroxyzine has been shown experimentally to have antispasmodic properties, apparently mediated through interference with the mechanism that responds to spasmogenic agents such as serotonin, acetylcholine, and histamine.
Antihistaminic effects have been demonstrated experimentally and confirmed clinically.
An antiemetic effect, both by the apomorphine test and the veriloid test, has been demonstrated. Pharmacological and clinical
studies indicate that hydroxyzine in therapeutic dosage does not increase gastric secretion or acidity and in most cases provides mild antisecretory benefits.
Hydroxyzine Indications and Usage
The total management of anxiety, tension, and psychomotor agitation in conditions of emotional stress requires in most instances a combined approach of psychotherapy and chemotherapy. Hydroxyzine has been found to be particularly useful for this latter phase of therapy in its ability to render the disturbed patient more amenable to psychotherapy in long term treatment of the psychoneurotic and psychotic, although it should not be used as the sole treatment of psychosis or of clearly demonstrated cases of depression. Hydroxyzine is also useful in alleviating the manifestations of anxiety and tension as in the preparation for dental procedures and in acute emotional problems. It has also been recommended for the management of anxiety associated with organic disturbances and as adjunctive therapy in alcoholism and allergic conditions with strong emotional overlay, such as in asthma, chronic urticaria, and pruritus.
Hydroxyzine hydrochloride intramuscular solution is useful in treating the following types of patients when intramuscular administration is indicated:
1. The acutely disturbed or hysterical patient.
2. The acute or chronic alcoholic with anxiety withdrawal symptoms or delirium tremens.
3. As pre-and postoperative and pre- and postpartum adjunctive medication to permit reduction in narcotic dosage, allay anxiety and control emesis.
Hydroxyzine hydrochloride has also demonstrated effectiveness in controlling nausea and vomiting, excluding nausea and vomiting of pregnancy. (See CONTRAINDICATIONS).
In prepartum states, the reduction in narcotic requirement effected by hydroxyzine is of particular benefit to both mother and neonate. Hydroxyzine benefits the cardiac patient by its ability to allay the associated anxiety and apprehension attendant to certain types of heart disease. Hydroxyzine is not known to interfere with the action of digitalis in any way and may be used concurrently with this agent.
The effectiveness of hydroxyzine in long term use, that is, more than 4 months, has not been assessed by systematic clinical studies.
The physician should reassess periodically the usefulness of the drug for the individual patient.
Contraindications
Hydroxyzine hydrochloride intramuscular solution is intended only for intramuscular administration and should not, under any circumstances, be injected subcutaneously, intra-arterially or intravenously.
This drug is contraindicated for patients who have shown a previous hypersensitivity to it.
Hydroxyzine, when administered to the pregnant mouse, rat, and rabbit, induced fetal abnormalities in the rat at doses substantially above the human therapeutic range. Clinical data in human beings are inadequate to establish safety in early pregnancy. Until such data are available, hydroxyzine is contraindicated in early pregnancy.
Precautions
THE POTENTIATING ACTION OF HYDROXYZINE MUST BE CONSIDERED WHEN THE DRUG IS USED IN CONJUNCTION WITH CENTRAL NERVOUS SYSTEM DEPRESSANTS SUCH AS NARCOTICS, BARBITURATES AND ALCOHOL.
Rarely, cardiac arrests and death have been reported in association with the combined use of hydroxyzine hydrochloride IM and other CNS depressants. Therefore, when central nervous system depressants are administered concomitantly with hydroxyzine their dosage should be reduced up to 50 percent. The efficacy of hydroxyzine as adjunctive pre- and postoperative
sedative medication has also been well established, especially as regards its ability to allay anxiety, control emesis, and reduce the amount of narcotic required.
HYDROXYZINE MAY POTENTIATE NARCOTICS AND BARBITURATES, so their use in preanesthetic adjunctive therapy should be modified on an individual basis. Atropine and other belladonna alkaloids are not affected by the drug. When hydroxyzine is used preoperatively or prepartum, narcotic requirements may be reduced as much as 50 percent. Thus, when
50 mg of hydroxyzine hydrochloride intramuscular solution is employed, meperidine dosage may be reduced from 100 mg to 50 mg. The administration of meperidine may result in severe hypotension in the postoperative patient or any individual whose ability to maintain blood pressure has been compromised by a depleted blood volume. Meperidine should be used with great caution and in reduced dosage in patients who are receiving other pre- and/or postoperative medications and in whom there is a risk of respiratory depression, hypotension, and profound sedation or coma occurring. Before using any medications concomitant with hydroxyzine, the manufacturer's prescribing information should be read carefully. Since drowsiness may occur with the use of this drug, patients should be warned of this possibility and cautioned against driving a car or operating dangerous machinery while taking this drug. As with all intramuscular preparations, hydroxyzine hydrochloride intramuscular solution should be injected well within the body of a relatively large muscle. Inadvertent subcutaneous injection may result in significant tissue damage.
Adults: The preferred site is the upper outer quadrant of the buttock, (i.e., the gluteus maximus), or the mid-lateral thigh.
Children: It is recommended that intramuscular injections be given preferably in the mid-lateral muscles of the thigh. In infants and small children the periphery of the upper outer quadrant of the gluteal region should be used only when necessary, such as in burn patients, in order to minimize the possibility of damage to the sciatic nerve. The deltoid area should be used only if well developed such as in certain adults and older children, and then only with caution to avoid radial nerve injury. Intramuscular injections should not be made into the lower and mid-third of the upper arm. As with all intramuscular injections, aspiration is necessary to help avoid inadvertent injection into a blood vessel.
Geriatric Use: A determination has not been made whether controlled clinical studies of hydroxyzine hydrochloride included sufficient numbers of subjects aged 65 and over to define a difference in response from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy. The extent of renal excretion of hydroxyzine hydrochloride has not been determined. Because elderly patients are more likely to have
decreased renal function, care should be taken in dose selections. Sedating drugs may cause confusion and over-sedation in the elderly; elderly patients generally should be started on low doses of hydroxyzine hydrochloride and observed closely.
Side Effects
Therapeutic doses of hydroxyzine seldom produce impairment of mental alertness. However, drowsiness may occur; if so, it is usually transitory and may disappear in a few days of continued therapy or upon reduction of the dose. Dryness of the mouth may be encountered at higher doses. Extensive clinical use has substantiated the absence of toxic effects on the liver or bone marrow when administered in the recommended doses for over four years of uninterrupted therapy. The absence of adverse effects has been further demonstrated in experimental studies in which excessively high doses were administered. Involuntary motor activity, including rare instances of tremor and convulsions, has been reported, usually with doses considerably higher than those recommended. Continuous therapy with over one gram per day has been employed in some patients without these effects having been encountered.
Dosage and Administration
The recommended dosages for hydroxyzine hydrochloride intramuscular solution are:
| For adult psychiatric and emotional emergencies, including acute alcholism |
IM: 50-100 mg stat., and q. 4-6h., p.r.n. |
| Nausea and vomiting excluding nausea and vomiting of pregnancy |
Adults:25-200 mg IM Children: 0.5 mg/lb body weight IM |
| Pre- and postoperative adjunctive medication |
Adults:25-200 mg IM Children: 0.5 mg/lb body weight IM |
| Pre-and postpartum adjunctive therapy |
25-100 mg IM |
How Supplied
| Product No. |
Strength |
Size |
|
| NDC 0517-4201-25 |
25 mg/mL |
1 mL Single Dose Vial |
Boxes of 25 |
| NDC 0517-5601-25 | 50 mg/mL | 1 mL Single Dose Vial | Boxes of 25 |
| NDC 0517-5602-25 | 50 mg/mL | 2 mL Single Dose Vial | Boxes of 25 |
| NDC 0517-5610-25 | 50 mg/mL | 10 mL Multi Dose Vial | Boxes of 25 |
AMERICAN
REGENT
LABORTORIES, INC.
Sample Package Label
HydroxyzineHydroxyzine INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||