Ibuprofen
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- IBUPROFEN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- IBUPROFEN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
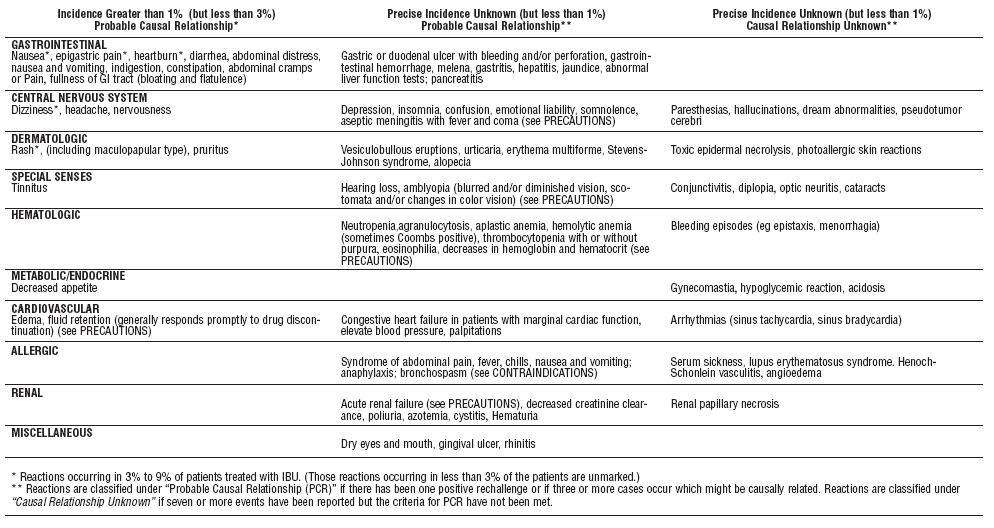
- IBUPROFEN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
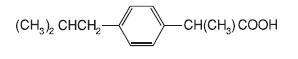
IBUPROFEN DESCRIPTION
CLINICAL PHARMACOLOGY
ADVERSE REACTIONS). Ibuprofen may be well toleratedin some patients who have had gastrointestinal side effectswith aspirin, but these patients when treated with IBU tablets shouldbe carefully followed for signs and symptoms of gastrointestinalulceration and bleeding. Although it is not definitely known whetheribuprofen causes less peptic ulceration than aspirin, in one studyinvolving 885 patients with rheumatoid arthritis treated for up to oneyear, there were no reports of gastric ulceration with ibuprofenwhereas frank ulceration was reported in 13 patients in the aspiringroup (statistically significant p<.001).
ADVERSE REACTIONS) and CNS side effects.
INDICATIONS & USAGE
WARNINGS).IBUPROFEN CONTRAINDICATIONS
WARNINGS, Anaphylactoid Reactions,andPRECAUTIONS, Preexisting Asthma).
WARNINGS).
WARNINGS
CARDIOVASCULAR EFFECTSCardiovascular Thrombotic Events
WARNINGS).
CONTRAINDICATIONS).
Hypertension
Congestive Heart Failure and Edema
Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation
Renal Effects
Advanced Renal Disease
Anaphylactoid Reactions
CONTRAINDICATIONSandPRECAUTIONS, Preexisting Asthma).Emergency help should be sought in cases where an anaphylactoidreaction occurs.
Skin Reactions
Pregnancy
PRECAUTIONS
GeneralHepatic effects
Hematological effects
Preexisting asthma
Ophthalmological effects.
Aseptic Meningitis
INFORMATION FOR PATIENTS
WARNINGS,Cardiovascular Effects).
WARNINGS,Gastrointestinal Effects-Risk of Ulceration,Bleeding and Perforation).
WARNINGS).
LABORATORY TESTS
DRUG INTERACTIONS
ACE-inhibitors:Reports suggest that NSAIDs may diminish the antihypertensiveeffect of ACE-inhibitors. This interaction should be given considerationin patients taking NSAIDs concomitantly with ACE-inhibitors.Aspirin
Diuretics
Lithium
Methotrexate
Warfarin-type anticoagulants
H-2 Antagonists
PREGNANCY
Teratogenic effects: Pregnancy Category CNonteratogenic effects
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
IBUPROFEN ADVERSE REACTIONS
OVERDOSAGE
DOSAGE & ADMINISTRATION
Rheumatoid arthritis and osteoarthritis, including flare-ups ofchronic disease:
Mild to moderate pain:
Dysmenorrhea:
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

IbuprofenIbuprofen TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!