Icy Hot
Icy Hot
FULL PRESCRIBING INFORMATION
ICYHOT
®
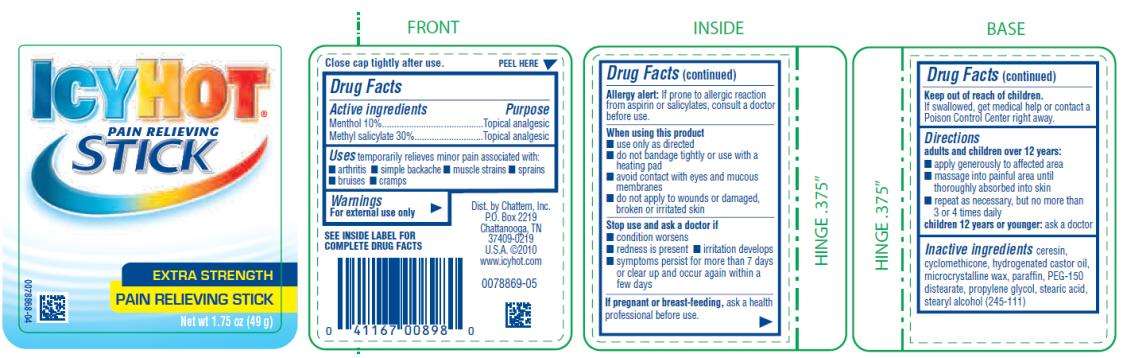
PAIN RELIEVING STICK
Drug Facts
Menthol 10%
Methyl salicylate 30%
Topical analgesic
temporarily relieves minor pain associated with:
- arthritis
- simple backache
- muscle strains
- sprains
- bruises
- cramps
For external use only
SEE INSIDE LABEL FOR COMPLETE DRUG FACTS
Allergy Alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
- use only as directed
- do not bandage tightly or use with a heating pad
- avoid contact with eyes or mucous membranes
- do not apply to wounds or damaged, broken or irritated skin
- condition worsens
- redness is present
- irritation develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
ask a health care professional before use.
If swallowed, get medical help or contact a Poison Control Center right away.
adults and children over 12 years:
- apply generously to affected area
- massage into painful area until thoroughly absorbed into skin
- repeat as necessary, but not more than 4 times daily
children 12 years or younger: ask a doctor
ceresin, cyclomethicone, hydrogenated castor oil, microcrystalline wax, paraffin, PEG-150 distearate, propylene glycol, stearic acid, stearyl alcohol (245-111)
ICYHOT
®
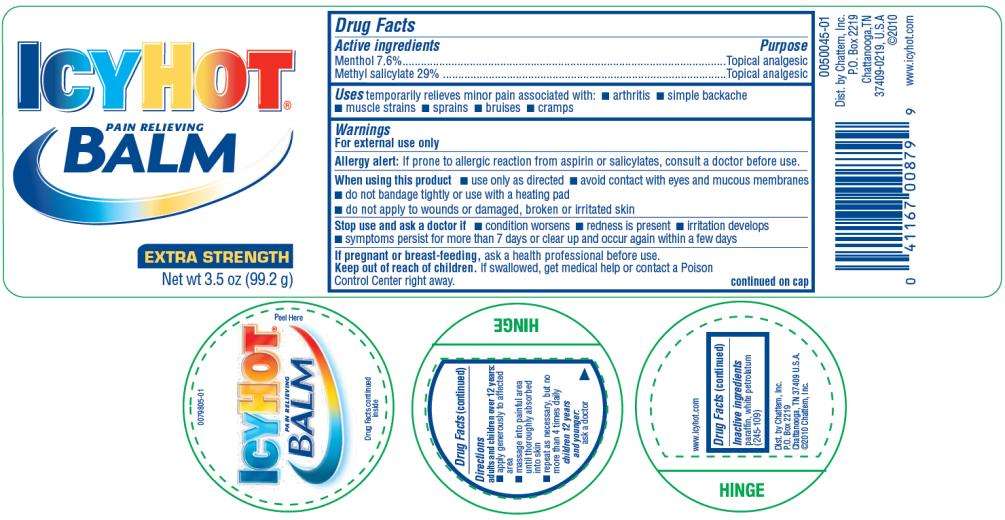
PAIN RELIEVING BALM
Drug Facts
Menthol 7.6%
Methyl salicylate 29%
Topical analgesic
temporarily relieves minor pain associated with:
- arthritis
- simple backache
- muscle strains
- sprains
- bruises
- cramps
For external use only
Allergy Alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
- use only as directed
- avoid contact with eyes or mucous membranes
- do not bandage tightly or use with a heating pad
- do not apply to wounds or damaged, broken or irritated skin
- condition worsens
- redness is present
- irritation develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
ask a health care professional before use.
If case of accidental ingestion, get medical help or contact a Poison Control Center right away.
continued on cap
adults and children over 12 years:
- apply generously to affected area
- massage into painful area until thoroughly absorbed into skin
- repeat as necessary, but not more than 4 times daily
children 12 years or younger: ask a doctor
paraffin, white petrolatum (245-109)
ICYHOT
®
PAIN RELIEVING CREAM
Drug Facts
Menthol 10%
Methyl salicylate 30%
Topical analgesic
temporarily relieves minor pain associated with:
- arthritis
- simple backache
- muscle strains
- sprains
- bruises
- cramps
For external use only
Allergy Alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
- use only as directed
- do not bandage tightly or use with a heating pad
- avoid contact with eyes or mucous membranes
- do not apply to wounds or damaged, broken or irritated skin
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
- redness is present
- irritation develops
ask a health care professional before use.
If case of accidental ingestion, get medical help or contact a Poison Control Center right away.
adults and children over 12 years:
- apply generously to affected area
- massage into painful area until thoroughly absorbed into skin
- repeat as necessary, but not more than 4 times daily
children 12 years or younger: ask a doctor
carbomer, cetyl esters, emulsifying wax, oleth-3 phosphate, stearic acid, triethanolamine, water (245-110)
Close cap tightly after use.
ICYHOT
®
PAIN RELIEVING
STICK
EXTRA STRENGTH
PAIN RELIEVING STICK
Net wt 1.
7
5 oz (
49
g)
ICYHOT
®
PAIN RELIEVING BALM
EXTRA STRENGTH
Net wt 3.5 oz (99.2 g)
ICYHOT
®
PAIN RELIEVING
CREAM
Net wt
1.
25 oz (
35.4
g)
EXTRA STRENGTH

Icy HotMenthol and Methyl Salicylate STICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Icy HotMenthol and Methyl Salicylate OINTMENT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Icy HotMenthol and Methyl Salicylate CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||