Influenza A (H1N1) 2009 Monovalent Vaccine
Novartis Vaccines and Diagnostics Ltd
HIGHLIGHTS OF PRESCRIBING INFORMATION INDICATIONS AND USAGE Influenza A (H1N1) 2009 Monovalent Vaccine is an inactivated influenza virus vaccine indicated for active immunization of persons 4 years of age and older against influenza disease caused by pandemic (H1N1) 2009 virus (1). DOSAGE AND ADMINISTRATION Based on currently available information the vaccination regimen is as follows: Children 4 through 9 years of age: Two 0.5-mL intramuscular injections approximately 1 month apart (2.2) Children 10 through 17 years of age: A single 0.5-mL intramuscular injection (2.2) Adults 18 years of age and older: A single 0.5-mL intramuscular injection (2.2) DOSAGE FORMS AND STRENGTHSInfluenza A (H1N1) 2009 Monovalent Vaccine, a sterile suspension for intramuscular injection, is supplied in two presentations: Prefilled single dose syringe, 0.5-mL. Thimerosal, a mercury derivative used during manufacture, is removed by subsequent purification steps to a trace amount (≤ 1 mcg mercury per 0.5-mL dose) (3, 11) Multidose vial, 5-mL. Contains thimerosal, a mercury derivative (25 mcg mercury per 0.5-mL dose). Thimerosal is added as preservative. (3,11) CONTRAINDICATIONS History of systemic hypersensitivity reactions to egg proteins, or any other component of Influenza A (H1N1) 2009 Monovalent Vaccine, or life-threatening reactions to previous influenza vaccinations. (4, 11) WARNINGS AND PRECAUTIONS If Guillain-Barré syndrome has occurred within 6 weeks of receipt of prior influenza vaccine, the decision to give Influenza A (H1N1) 2009 Monovalent Vaccine should be based on careful consideration of the potential benefits and risks. (5.1) Immunocompromised persons may have a reduced immune response to Influenza A (H1N1) 2009 Monovalent Vaccine. (5.2) Side EffectsAdverse Reaction information is based on studies conducted with seasonal trivalent Influenza Virus Vaccine manufactured by Novartis (FLUVIRIN). The most frequently reported adverse reactions are mild hypersensitivity reactions (such as rash), local reactions at the injection site, and influenza-like symptoms. (6) To report SUSPECTED ADVERSE REACTIONS contact Novartis Vaccines at 1-800-244-7668, or VAERS at 1-800-822-7967 and www.vaers.hhs.gov. DRUG INTERACTIONS Do not mix with any other vaccine in the same syringe or vial. (7.1) Immunosuppressive therapies may reduce immune response to Influenza A (H1N1) 2009 Monovalent Vaccine. (7.2) USE IN SPECIFIC POPULATIONS Safety and effectiveness of Influenza A (H1N1) 2009 Monovalent Vaccine have not been established in pregnant women, nursing mothers or children less than 4 years of age. (8.1, 8.3, 8.4) Antibody responses to the trivalent seasonal Influenza Virus Vaccine manufactured by Novartis (FLUVIRIN) were lower in the geriatric population than in younger subjects. (8.5)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 Influenza A (H1N1) 2009 Monovalent Vaccine Indications and Usage
- 2 Dosage and Administration
- 3 DOSAGE FORMS AND STRENGTHS
- 4 INFLUENZA A (H1N1) 2009 MONOVALENT VACCINE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 INFLUENZA A (H1N1) 2009 MONOVALENT VACCINE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 11 INFLUENZA A (H1N1) 2009 MONOVALENT VACCINE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 Indications and Usage
Influenza A (H1N1) 2009 Monovalent Vaccine is an inactivated influenza virus vaccine indicated for immunization of persons 4 years of age and older against influenza disease caused by pandemic (H1N1) 2009 virus.
2 Dosage and Administration
2.1 Preparation for Administration
Inspect Influenza A (H1N1) 2009 Monovalent Vaccine syringes and multidose vials visually for particulate matter and/or discoloration prior to administration. If either of these conditions exists, the vaccine should not be administered.
Shake the syringe vigorously before administering the vaccine and shake the multidose vial preparation each time before withdrawing a dose of vaccine.
Between uses, return the multidose vial to the recommended storage conditions between 2º and 8ºC (36º and 46ºF). Do not freeze. Discard if the vaccine has been frozen.
A separate syringe and needle or a sterile disposable unit should be used for each injection to prevent transmission of infectious agents from one person to another. Needles should be disposed of properly and not recapped.
It is recommended that small syringes (0.5-mL or 1-mL) should be used to minimize any product loss.
2.2 Recommended Dose and Schedule
Clinical studies are ongoing with Influenza A (H1N1) 2009 Monovalent Vaccine to determine the optimal dosage, number of doses and schedule.
Available data show that children 9 years of age and younger are largely serologically naïve to the pandemic (H1N1) 2009 virus (15.1). Based upon these data Influenza A (H1N1) 2009 Monovalent Vaccine should be administered as follows:
Children (4 to 17 years of age):
Children 4 through 9 years of age should receive two 0.5mL doses by intramuscular injection approximately 1 month apart.
Children 10 through 17 years of age should receive a single 0.5-mL intramuscular injection.
The needle size may range from 7/8 to 1¼ inches, depending on the size of the child’s deltoid muscle, and should be of sufficient length to penetrate the muscle tissue. The anterolateral thigh can be used, but the needle should be longer, usually 1 inch.
The vaccine should not be injected in the gluteal region or areas where there may be a major nerve trunk.
Adults (18 years of age and older):
Influenza A (H1N1) 2009 Monovalent Vaccine should be administered as a single 0.5-mL intramuscular injection preferably in the region of the deltoid muscle of the upper arm.
A needle of ≥1 inch is preferred because needles <1 inch might be of insufficient length to penetrate muscle tissue in certain adults.
The vaccine should not be injected in the gluteal region or areas where there may be a major nerve trunk.
3 DOSAGE FORMS AND STRENGTHS
Influenza A (H1N1) 2009 Monovalent Vaccine is a sterile suspension for intramuscular injection. [see DESCRIPTION (11) for the complete list of ingredients]
Influenza A (H1N1) 2009 Monovalent Vaccine is available in two presentations:
1) Prefilled single dose syringe, 0.5-mL. Thimerosal, a mercury derivative used during manufacture, is removed by subsequent purification steps to a trace amount (≤ 1 mcg mercury per 0.5-mL dose).
2) Multidose vial, 5-mL. Contains thimerosal, a mercury derivative, added as a preservative. Each 0.5-mL dose from the multidose vial contains 25 mcg mercury.
4 CONTRAINDICATIONS
4.1 Hypersensitivity
Influenza A (H1N1) 2009 Monovalent Vaccine should not be administered to anyone with known systemic hypersensitivity reactions to egg proteins (eggs or egg products), or to any component of Influenza A (H1N1) 2009 Monovalent Vaccine, or who has had a life-threatening reaction to previous influenza vaccinations [see DESCRIPTION (11)].
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome
If Guillain-Barré syndrome has occurred within 6 weeks of receipt of prior influenza vaccine, the decision to give Influenza A (H1N1) 2009 Monovalent Vaccine should be based on careful consideration of the potential benefits and risks.
5.2 Altered Immunocompetence
If Influenza A (H1N1) 2009 Monovalent Vaccine is administered to immunocompromised persons, including individuals receiving immunosuppressive therapy, the expected immune response may not be obtained.
5.3 Preventing and Managing Allergic Reactions
Prior to administration of any dose of Influenza A (H1N1) 2009 Monovalent Vaccine, the healthcare provider should review the patient’s prior immunization history for possible adverse events, to determine the existence of any contraindication to immunization with Influenza A (H1N1) 2009 Monovalent Vaccine and to allow an assessment of benefits and risks. Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine.
5.4 Limitations of Vaccine Effectiveness
Vaccination with Influenza A (H1N1) 2009 Monovalent Vaccine may not protect all individuals.
6 ADVERSE REACTIONS
Novartis’ Influenza A (H1N1) 2009 Monovalent Vaccine and seasonal trivalent Influenza Virus Vaccine (FLUVIRIN®) are manufactured by the same process. The data in this section were obtained from clinical studies and postmarketing experience with FLUVIRIN.
6.1 Overall Adverse Reaction Profile
Serious allergic reactions, including anaphylactic shock, have been observed in individuals receiving FLUVIRIN during postmarketing surveillance.
6.2 Clinical Trial Experience
Adverse event information from clinical trials provides a basis for identifying adverse events that appear to be related to vaccine use and for approximating the rates of these events. However, because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine, and may not reflect rates observed in clinical practice.
Adult and Geriatric Subjects
Safety data were collected in a total of 2768 adult and geriatric subjects (18 years of age and older) who have received FLUVIRIN in 29 clinical studies since 1982.
In 9 clinical studies since 1997, among 1261 recipients of FLUVIRIN, 745 (59%) were women; 1211 (96%) were White, 23 (2%) Asian, 15 (1%) Black and 12 (1%) other; 370 (29%) of subjects were elderly (≥65 years of age). All studies have been conducted in the UK, apart from a study run in the US in 2005-2006 where FLUVIRIN was used as a comparator for an unlicensed vaccine.
After vaccination, the subjects were observed for 30 minutes for hypersensitivity or other immediate reactions. Subjects were instructed to complete a diary card for three days following immunization (i.e. Day 1 to 4) to collect local and systemic reactions (see Tables 1 and 2). All local and systemic adverse events were considered to be at least possibly related to the vaccine. Local and systemic reactions mostly began between day 1 and day 2. The overall adverse events reported in clinical trials since 1998 in at least 5% of the subjects are summarized in Table 3.
| 1998-1999 *§ | 1999-2000 *§ | 2000-2001 *§ | ||||
|---|---|---|---|---|---|---|
| 18-64 yrs | ≥ 65 yrs | 18-64 yrs | ≥ 65 yrs | 18-64 yrs | ≥ 65 yrs | |
| N = 66 | N = 44 | N = 76 | N = 34 | N = 75 | N = 35 | |
|
Results reported to the nearest whole percent; Fever defined as >38°C |
||||||
|
– not reported |
||||||
|
* Solicited adverse events in the first 72 hours after administration of FLUVIRIN |
||||||
|
§ Solicited adverse events reported by COSTART preferred term |
||||||
|
^ Solicited adverse events reported by MEDDRA preferred term |
||||||
| Local Adverse Events | ||||||
| Pain | 16 (24%) | 4 (9%) | 16 (21%) | - | 9 (12%) | - |
| Mass | 7 (11%) | 1 (2%) | 4 (5%) | - | 8 (11%) | 1 (3%) |
| Inflammation | 5 (8%) | 2 (5%) | 6 (8%) | - | 7 (9%) | 1 (3%) |
| Ecchymosis | 4 (6%) | 1 (2%) | 3 (4%) | 1 (3%) | 4 (5%) | - |
| Edema | 2 (3%) | 1 (2%) | 1 (1%) | 2 (6%) | 3 (4%) | 1 (3%) |
| Reaction | 2 (3%) | - | 2 (3%) | - | 4 (5%) | 1 (3%) |
| Hemorrhage | - | - | 1 (1%) | - | - | - |
| Systemic Adverse Events | ||||||
| Headache | 7 (11%) | 1 (2%) | 17 (22%) | 3 (9%) | 4 (5%) | - |
| Fatigue | 3 (5%) | 2 (5%) | 4 (5%) | 1 (3%) | 3 (4%) | - |
| Malaise | 2 (3%) | 1 (2%) | 2 (3%) | 1 (3%) | 1 (1%) | - |
| Myalgia | 1 (2%) | - | 2 (3%) | - | - | - |
| Fever | 1 (2%) | - | 1 (1%) | - | - | - |
| Arthralgia | - | 1 (2%) | - | 1 (3%) | - | - |
| Sweating | - | - | 3 (4%) | - | 1 (1%) | 1 (3%) |
| 2001-2002 *^ | 2002-2003 *^ | 2004-2005* ^ | ||||
| 18-64 yrs | ≥ 65 yrs | 18-64 yrs | ≥ 65 yrs | 18-64 yrs | ≥ 65 yrs | |
| N = 75 | N = 35 | N = 107 | N = 88 | N = 74 | N = 61 | |
| Local Adverse Events | ||||||
| Pain | 12 (16%) | 1 (3%) | 14 (13%) | 7 (8%) | 15 (20%) | 9 (15%) |
| Mass | 4 (5%) | 1 (3%) | - | - | - | - |
| Ecchymosis | 2 (3%) | - | 3 (3%) | 3 (3%) | 2 (3%) | 1 (2%) |
| Edema | 2 (3%) | 1 (3%) | 6 (6%) | 2 (2%) | - | - |
| Erythema | 5 (7%) | - | 11 (10%) | 5 (6%) | 16 (22%) | 5 (8%) |
| Swelling | - | - | - | - | 11 (15%) | 4 (7%) |
| Reaction | - | - | 2 (2%) | - | - | - |
| Induration | - | - | 14 (13%) | 3 (3%) | 11 (15%) | 1 (2%) |
| Pruritus | - | - | 1 (1%) | - | - | - |
| Systemic Adverse Events | ||||||
| Headache | 8 (11%) | 1 (3%) | 12 (11%) | 9 (10%) | 14 (19%) | 3 (5%) |
| Fatigue | 1 (1%) | 1 (3%) | - | - | 5 (7%) | 2 (3%) |
| Malaise | 3 (4%) | - | 3 (3%) | 4 (5%) | 1 (1%) | 1 (2%) |
| Myalgia | 3 (4%) | - | 5 (5%) | 3 (3%) | 8 (11%) | 1 (2%) |
| Fever | - | - | - | 1 (1%) | - | - |
| Arthralgia | - | - | 2 (2%) | - | 1 (1%) | - |
| Sweating | 3 (4%) | 1 (3%) | - | 2 (2%) | - | - |
| Shivering | - | - | - | 1 (1%) | - | - |
| 2005-2006 US Trial | |
|---|---|
| FLUVIRIN | |
| N = 304 | |
|
Results reported to the nearest whole percent |
|
|
– not reported |
|
| Local Adverse Events | |
| Pain | 168 (55%) |
| Erythema | 48 (16%) |
| Ecchymosis | 22 (7%) |
| Induration | 19 (6%) |
| Swelling | 16 (5%) |
| Systemic Adverse Events | |
| Headache | 91 (30%) |
| Myalgia | 64 (21%) |
| Malaise | 58 (19%) |
| Fatigue | 56 (18%) |
| Sore throat | 23 (8%) |
| Chills | 22 (7%) |
| Nausea | 21 (7%) |
| Arthralgia | 20 (7%) |
| Sweating | 17 (6%) |
| Cough | 18 (6%) |
| Wheezing | 4 (1%) |
| Chest tightness | 4 (1%) |
| Other difficulties breathing | 3 (1%) |
| Facial edema | - |
| 1998-1999§ | 1999-2000§ | 2000-2001§ | ||||||
|---|---|---|---|---|---|---|---|---|
| 18-64 yrs | ≥ 65 yrs | 18-64 yrs | ≥ 65 yrs | 18-64 yrs | ≥ 65 yrs | |||
| N = 66 | N = 44 | N = 76 | N = 34 | N = 75 | N = 35 | |||
|
Results reported to the nearest whole percent; Fever defined as >38°C |
||||||||
|
– not reaching the cut-off of 5% |
||||||||
|
§ Solicited adverse events reported by COSTART preferred term |
||||||||
|
^ Solicited adverse events reported by MEDDRA preferred term |
||||||||
| Adverse Events | ||||||||
| Fatigue | 8 (12%) | 2 (5%) | 8 (11%) | 2 (6%) | 5 (7%) | - | ||
| Back pain | 4 (6%) | 3 (7%) | - | - | - | - | ||
| Cough increased | 2 (3%) | 2 (5%) | - | - | - | - | ||
| Ecchymosis | 4 (6%) | 1 (2%) | 4 (5%) | 1 (3%) | 5 (7%) | - | ||
| Fever | 3 (5%) | - | - | - | - | - | ||
| Headache | 12 (18%) | 5 (11%) | 22 (29%) | 5 (15%) | 14 (19%) | 2 (6%) | ||
| Infection | 3 (5%) | 2 (5%) | - | - | - | - | ||
| Malaise | 4 (6%) | 4 (9%) | 4 (5%) | 1 (3%) | - | - | ||
| Migraine | 4 (6%) | 1 (2%) | - | - | - | - | ||
| Myalgia | 4 (6%) | 1 (2%) | - | - | - | - | ||
| Sweating | 5 (8%) | 1 (2%) | - | - | - | - | ||
| Rhinitis | 3 (5%) | 1 (2%) | - | - | 5 (7%) | 2 (6%) | ||
| Pharingitis | 6 (9%) | 1 (2%) | 10 (13%) | - | 6 (8%) | - | ||
| Arthralgia | - | - | - | 2 (6%) | - | - | ||
| Injection site pain | 16 (24%) | 4 (9%) | 16 (21%) | - | 9 (12%) | - | ||
| Injection site ecchymosis | 4 (6%) | 1 (2%) | - | - | 4 (5%) | - | ||
| Injection site mass | 7 (11%) | 1 (2%) | 4 (5%) | - | 8 (11%) | 1 (3%) | ||
| Injection site edema | - | - | 1 (1%) | 2 (6%) | - | - | ||
| Injection site inflammation | 5 (8%) | 2 (5%) | 6 (8%) | - | 7 (9%) | 1 (3%) | ||
| Injection site reaction | - | - | - | - | 4 (5%) | 1 (3%) | ||
| 2001-2002 ^ | 2002-2003 ^ | 2004-2005 ^ | ||||||
| 18-64 yrs | ≥ 65 yrs | 18-64 yrs | ≥ 65 yrs | 18-64 yrs | ≥ 65 yrs | |||
| N = 75 | N = 35 | N = 107 | N = 88 | N = 74 | N = 61 | |||
| Adverse Events | ||||||||
| Fatigue | 5 (7%) | 4 (11%) | 11 (10%) | 8 (9%) | 4 (5%) | 2 (3%) | ||
| Hypertension | - | - | 1 (1%) | 4 (5%) | - | - | ||
| Rinorrhea | - | - | 2 (2%) | 5 (6%) | - | - | ||
| Headache | 20 (27%) | 2 (6%) | 35 (33%) | 18 (20%) | 12 (16%) | 1 (2%) | ||
| Malaise | 6 (8%) | 1 (3%) | 13 (12%) | 8 (9%) | - | - | ||
| Myalgia | 4 (5%) | 1 (3%) | 10 (9%) | 4 (5%) | - | - | ||
| Sweating | 3 (4%) | 3 (9%) | 2 (2%) | 5 (6%) | - | - | ||
| Rhinitis | 4 (5%) | - | - | - | - | - | ||
| Pharingitis | - | - | - | - | 6 (8%) | - | ||
| Arthralgia | - | - | 5 (5%) | 4 (5%) | - | - | ||
| Sore throat | 4 (5%) | 1 (3%) | 5 (5%) | 4 (5%) | - | - | ||
| Injection site pain | 13 (17%) | 3 (9%) | 14 (13%) | 7 (8%) | 6 (8%) | 2 (3%) | ||
| Injection site ecchymosis | 4 (5%) | 1 (3%) | 4 (4%) | 4 (5%) | - | - | ||
| Injection site erythema | 5 (7%) | 2 (6%) | 11 (10%) | 5 (6%) | 4 (5%) | - | ||
| Injection site mass | 4 (5%) | 1 (3%) | - | - | - | - | ||
| Injection site edema | - | - | 6 (6%) | 2 (2%) | 4 (5%) | 1 (2%) | ||
| Injection site induration | - | - | 14 (13%) | 3 (3%) | 7 (9%) | - | ||
Adults (18 to 64 years of age)
In adult subjects, solicited local adverse events occurred with similar frequency in all trials. The most common solicited adverse events occurring in the first 96 hours after administration (Tables 1 and 2) were associated with the injection site (such as pain, erythema, mass, induration and swelling) but were generally mild/moderate and transient. The most common solicited systemic adverse events were headache and myalgia.
The most common overall events in adult subjects (18-64 years of age) were headache, fatigue, injection site reactions (pain, mass, erythema, and induration) and malaise (Table 3).
Geriatric Subjects (65 years of age and older)
In geriatric subjects, solicited local and systemic adverse events occurred less frequently than in adult subjects. The most common solicited local and systemic adverse events were injection site pain, and headache (Tables 1 and 2). All were considered mild/moderate and were transient.
The most common overall events in elderly subjects (≥65 years of age) were headache and fatigue.
Only 11 serious adverse events in adult and geriatric subjects (18 years and older) have been reported to date from all the trials performed. These serious adverse events were a minor stroke experienced by a 67 year old subject 14 days after vaccination (1990), death of an 82 year old subject 35 days after vaccination (1990) in very early studies; death of a 72 year old subject 19 days after vaccination (1998-1999), a hospitalization for hemorrhoidectomy of a 38 year old male subject (1999-2000), a severe respiratory tract infection experienced by a 74 year old subject 12 days after vaccination (2002-2003), a planned transurethral resection of the prostate in a subject with prior history of prostatism (2004-2005), two cases of influenza (2005-2006), a drug overdose (2005-2006), cholelithiasis (2005-2006) and a nasal septal operation (2005-2006). None of these events were considered causally related to vaccination.
Clinical Trial Experience in Pediatric Subjects
In 1987 a clinical study was carried out in 38 ‘at risk’ children aged between 4 and 12 years (17 females and 21 males). To record the safety of FLUVIRIN, participants recorded their symptoms on a diary card during the three days after vaccination and noted any further symptoms they thought were attributable to the vaccine. The only reactions recorded were tenderness at the site of vaccination in 21% of the participants on day 1, which was still present in 16% on day 2 and 5% on day 3. In one child, the tenderness was also accompanied by redness at the site of injection for two days. The reactions were not age-dependent and there was no bias towards the younger children.
Three clinical studies were carried out between 1995 and 2004 in a total of 520 pediatric subjects (age range 6 - 47 months). Of these, 285 healthy subjects plus 41 ‘at risk’ subjects received FLUVIRIN. No serious adverse events were reported.
FLUVIRIN and Influenza A (H1N1) 2009 Monovalent Vaccine should only be used for the immunization of persons aged 4 years and over.
6.3 Postmarketing Experience
The following additional adverse reactions have been reported during post-approval use of FLUVIRIN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure. Adverse events described here are included because: a) they represent reactions which are known to occur following immunizations generally or influenza immunizations specifically; b) they are potentially serious; or c) the frequency of reporting.
- Body as a whole: Local injection site reactions (including pain, pain limiting limb movement, redness, swelling, warmth, ecchymosis, induration), hot flashes/flushes; chills; fever; malaise; shivering; fatigue; asthenia; facial edema.
- Immune system disorders: Hypersensitivity reactions (including throat and/or mouth edema). In rare cases, hypersensitivity reactions have lead to anaphylactic shock and death.
- Cardiovascular disorders: Vasculitis (in rare cases with transient renal involvement), syncope shortly after vaccination.
- Digestive disorders: Diarrhea; nausea; vomiting; abdominal pain.
- Blood and lymphatic disorders: Local lymphadenopathy; transient thrombocytopenia.
- Metabolic and nutritional disorders: Loss of appetite.
- Musculoskeletal: Arthralgia; myalgia; myasthenia.
- Nervous system disorders: Headache; dizziness; neuralgia; paraesthesia; confusion; febrile convulsions; Guillain-Barré Syndrome; myelitis (including encephalomyelitis and transverse myelitis); neuropathy (including neuritis); paralysis (including Bell’s Palsy).
- Respiratory disorders: Dyspnea; chest pain; cough; pharyngitis; rhinitis.
- Skin and appendages: Stevens-Johnson syndrome; sweating; pruritus; urticaria; rash (including non-specific, maculopapular, and vesiculobulbous).
6.4 Other Side Effects Associated with Influenza Vaccination
Anaphylaxis has been reported after administration of FLUVIRIN. Although FLUVIRIN and Influenza A (H1N1) 2009 Monovalent Vaccine contain only a limited quantity of egg protein, this protein can induce immediate hypersensitivity reactions among persons who have severe egg allergy. Allergic reactions include hives, angioedema, allergic asthma, and systemic anaphylaxis [see CONTRAINDICATIONS (4)].
The 1976 swine influenza vaccine was associated with an increased frequency of Guillain-Barré syndrome (GBS). Evidence for a causal relation of GBS with subsequent vaccines prepared from other influenza viruses is unclear. If influenza vaccine does pose a risk, it is probably slightly more than 1 additional case/1 million persons vaccinated.
Neurological disorders temporally associated with influenza vaccination such as encephalopathy, optic neuritis/neuropathy, partial facial paralysis, and brachial plexus neuropathy have been reported.
Microscopic polyangiitis (vasculitis) has been reported temporally associated with influenza vaccination.
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Other Vaccines
There are no data to assess the concomitant administration of Influenza A (H1N1) 2009 Monovalent Vaccine with other vaccines. If Influenza A (H1N1) 2009 Monovalent Vaccine is to be given at the same time as another injectable vaccine(s), the vaccines should always be administered at different injection sites.
Influenza A (H1N1) 2009 Monovalent Vaccine should not be mixed with any other vaccine in the same syringe or vial.
7.2 Concurrent Use with Immunosuppressive Therapies
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs, and corticosteroids (used in greater than physiologic doses), may reduce the immune response to Influenza A (H1N1) 2009 Monovalent Vaccine.
8 USE IN SPECIFIC POPULATIONS
Novartis’ Influenza A (H1N1) 2009 Monovalent Vaccine and seasonal trivalent Influenza Virus Vaccine (FLUVIRIN) are manufactured by the same process. Available information for FLUVIRIN is provided in this section.
8.1 Pregnancy
Pregnancy Category C: Animal reproduction studies have not been conducted with Influenza A (H1N1) 2009 Monovalent Vaccine or FLUVIRIN. It is also not known whether Influenza A (H1N1) 2009 Monovalent Vaccine or FLUVIRIN can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Influenza A (H1N1) 2009 Monovalent Vaccine should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether FLUVIRIN or Influenza A (H1N1) 2009 Monovalent Vaccine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Influenza A (H1N1) 2009 Monovalent Vaccine is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness in pediatric subjects below the age of 4 years have not been established. [see ADVERSE REACTIONS (6) and CLINICAL STUDIES (14)]
8.5 Geriatric Use
Since 1997, of the total number of geriatric subjects (n = 397) in clinical studies of FLUVIRIN, 29% of adult subjects were 65 years and over, while 2.1% were 75 years and over.
Antibody responses were lower in the geriatric population than in younger subjects. Adverse events occurred less frequently in geriatric subjects (≥65 years) than in younger adults. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. [See ADVERSE REACTION (6) and CLINICAL STUDIES (14)].
11 DESCRIPTION
Influenza A (H1N1) 2009 Monovalent Vaccine is a sub-unit (purified surface antigen) influenza virus vaccine prepared from virus propagated in the allantoic cavity of embryonated hens’ eggs inoculated with a specific type of influenza virus suspension containing neomycin and polymyxin. The influenza virus strain is harvested and clarified by centrifugation and filtration prior to inactivation with betapropiolactone. The inactivated virus is concentrated and purified by zonal centrifugation. The surface antigens, hemagglutinin and neuraminidase, are obtained from the influenza virus particle by further centrifugation in the presence of nonylphenol ethoxylate, a process which removes most of the internal proteins. The nonylphenol ethoxylate is removed from the surface antigen preparation.
Influenza A (H1N1) 2009 Monovalent Vaccine is a homogenized, sterile, slightly opalescent suspension in a phosphate buffered saline. Influenza A (H1N1) 2009 Monovalent Vaccine is formulated to contain 15 mcg hemagglutinin (HA) per 0.5-mL dose of the following virus strain: A/California/7/2009 (H1N1)v-like virus.
The 0.5-mL prefilled syringe presentation is formulated without preservative. Thimerosal, a mercury derivative used during manufacturing, is removed by subsequent purification steps to a trace amount (≤ 1 mcg mercury per 0.5-mL dose).
The 5-mL multidose vial formulation contains thimerosal, a mercury derivative, added as a preservative. Each 0.5-mL dose from the multidose vial contains 25 mcg mercury.
Each dose from the multidose vial or from the prefilled syringe may also contain residual amounts of egg proteins (≤ 1 mcg ovalbumin), polymyxin (≤ 3.75 mcg), neomycin (≤ 2.5 mcg), betapropiolactone (not more than 0.5 mcg) and nonylphenol ethoxylate (not more than 0.015% w/v).
The multidose vial stopper and the syringe stopper/plunger do not contain latex.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Influenza illness and its complications follow infection with influenza viruses. Global surveillance of influenza identifies yearly antigenic variants. For example, since 1977, antigenic variants of influenza A (H1N1 and H3N2) viruses and influenza B viruses have been in global circulation. Specific levels of hemagglutination inhibition (HI) antibody titers post-vaccination with inactivated influenza virus vaccine have not been correlated with protection from influenza illness. In some human studies, antibody titer of ≥1:40 have been associated with protection from influenza illness in up to 50% of subjects [see REFERENCES (15.2, 15.3)].
Antibody against one influenza virus type or subtype confers limited or no protection against another. Furthermore, antibody to one antigenic variant of influenza virus might not protect against a new antigenic variant of the same type or subtype.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Neither FLUVIRIN nor the Influenza A (H1N1) 2009 Monovalent Vaccine have been evaluated for carcinogenic or mutagenic potential, or for impairment of fertility.
14 CLINICAL STUDIES
Novartis’ Influenza A (H1N1) 2009 Monovalent Vaccine and seasonal trivalent Influenza Virus Vaccine (FLUVIRIN) are manufactured by the same process. Data in this section were obtained in clinical studies conducted with FLUVIRIN.
Between 1982 and 1991, twelve clinical studies were conducted in healthy adult and geriatric subjects and one in children between 4 and 12 years of age who were considered to be ‘at risk’. Since 1991 an annual clinical study has been conducted in the UK in healthy adults aged 18 years or older. FLUVIRIN was also used as a control in a US clinical trial in adults (18-49 years of age). In all the trials, blood samples were taken prior to vaccination and approximately three weeks after vaccination to assess the immunogenic response to vaccination by measurement of anti-HA antibodies.
Three clinical studies were carried out between 1995 and 2004 in a total of 520 pediatric subjects (age range 6-48 months). Of these, 285 healthy subjects plus 41 ‘at risk’ pediatric subjects, received FLUVIRIN.
Influenza A (H1N1) 2009 Monovalent Vaccine should only be used for the immunization of persons aged 4 years and over.
14.1 Immunogenicity in Adults (18 to 64 years of age)
Tables 4 and 5 show the immunogenicity data for the adult age group. The seven clinical studies presented enrolled a total of 774 adult subjects. In the adult group, for all antigens (A/H1N1, A/H3N2 and B) at least one of the following point estimate criteria was met: the proportion of subjects with seroconversion (post-vaccination titer ≥1:40 from a pre-vaccination titer <1:10) or significant increase (at least a four-fold increase from pre-vaccination titer ≥1:10) in antibody titer was greater than 40%; the geometric mean titer (GMT) increase was >2.5; the proportion of subjects with a post-vaccination hemagglutination inhibition (HI) antibody titer ≥1:40 was greater than 70%.
| Year/Strain | No. of subjects | Seroconversion ∞ | HI titer ≥1:40¥ | ||||
|---|---|---|---|---|---|---|---|
| N | % | 95% CIΦ | N | % | 95% CIΦ | ||
|
∞ Seroconversion: proportion of subjects with either a post-vaccination HI titer ≥1:40 from a pre-vaccination titer <1:10 or at least a four-fold increase from pre-vaccination HI titer ≥1:10 in antibody titer. |
|||||||
|
¥ HI titer ≥1:40: proportion of subjects with a post-vaccination titer ≥ 1:40. |
|||||||
|
Φ 95% CI: 95% confidence interval |
|||||||
| 1998-1999 | |||||||
| A/H1N1 | 48 | 73 | (62, 83) | 50 | 76 | (65, 86) | |
| A/H3N2 | 66 | 43 | 65 | (54, 77) | 47 | 71 | (60, 82) |
| B | 42 | 64 | (52, 75) | 62 | 94 | (88, 100) | |
| 1999-2000 | |||||||
| A/H1N1 | 45 | 59 | (48, 70) | 50 | 66 | (55, 76) | |
| A/H3N2 | 76 | 51 | 67 | (57, 78) | 66 | 87 | (79, 94) |
| B | 53 | 70 | (59, 80) | 75 | 99 | (96, 100) | |
| 2000-2001 | |||||||
| A/H1N1 | 41 | 55 | (44, 67) | 41 | 55 | (44, 67) | |
| A/H3N2 | 74 | 45 | 61 | (50, 72) | 52 | 84 | (75, 92) |
| B | 50 | 68 | (57, 78) | 73 | 99 | (96, 100) | |
| 2001-2002 | |||||||
| A/H1N1 | 44 | 59 | (48, 70) | 48 | 64 | (53, 75) | |
| A/H3N2 | 75 | 46 | 61 | (50, 72) | 68 | 91 | (84, 97) |
| B | 42 | 56 | (45, 67) | 66 | 88 | (81, 95) | |
| 2002-2003 | |||||||
| A/H1N1 | 62 | 58 | (49, 68) | 73 | 69 | (60, 78) | |
| A/H3N2 | 106 | 72 | 68 | (59, 77) | 93 | 88 | (81, 94) |
| B | 78 | 74 | (65, 82) | 101 | 95 | (91, 99) | |
| 2004-2005 | |||||||
| A/H1N1 | 52 | 70 | (59, 80) | 66 | 89 | (80, 95) | |
| A/H3N2 | 74 | 60 | 81 | (70, 89) | 73 | 99 | (93, 100) |
| B | 57 | 77 | (66, 86) | 69 | 93 | (85, 98) | |
| 2005-2006 | |||||||
| A/H1N1 | 191 | 63 | (57, 68) | 296 | 98 | (95, 99) | |
| A/H3N2 | 303 | 273 | 90 | (86, 93) | 294 | 97 | (94, 99) |
| B | 213 | 70 | (65, 75) | 263 | 87 | (82, 90) | |
| Year/Strain | No. of subjects | Geometric Mean Titer (GMT) | |||
|---|---|---|---|---|---|
| Pre-vaccination | Post-vaccination | Fold Increase | (95% CI)* | ||
|
* 95% CI: 95% confidence interval |
|||||
| 1998-1999 | |||||
| A/H1N1 | 7.26 | 160.87 | 22.16 | (14.25, 34.46) | |
| A/H3N2 | 66 | 8.23 | 87.02 | 10.57 | (6.91, 16.16) |
| B | 20.97 | 231.07 | 110.2 | (6.90, 17.59) | |
| 1999-2000 | |||||
| A/H1N1 | 7.43 | 58.95 | 7.93 | (5.73, 10.97) | |
| A/H3N2 | 76 | 15.29 | 122.83 | 8.03 | (5.80, 11.13) |
| B | 25.70 | 254.76 | 9.91 | (6.97, 14.10) | |
| 2000-2001 | |||||
| A/H1N1 | 5.42 | 33.80 | 6.24 | (4.49, 8.69) | |
| A/H3N2 | 74 | 15.98 | 126.01 | 7.89 | (5.61, 11.09) |
| B | 26.24 | 308.25 | 11.75 | (7.73, 17.85) | |
| 2001-2002 | |||||
| A/H1N1 | 7.76 | 54.78 | 7.06 | (5.24, 9.52) | |
| A/H3N2 | 75 | 23.67 | 153.81 | 6.50 | (4.78, 8.84) |
| B | 19.91 | 107.53 | 5.40 | (3.95, 7.38) | |
| 2002-2003 | |||||
| A/H1N1 | 7.78 | 60.39 | 7.77 | (5.81, 10.39) | |
| A/H3N2 | 106 | 23.32 | 292.03 | 12.52 | (8.77, 17.87) |
| B | 30.20 | 314.11 | 10.40 | (7.54, 14.34) | |
| 2004-2005 | |||||
| A/H1N1 | 13 | 159 | 12 | (8.39, 17) | |
| A/H3N2 | 74 | 37 | 658 | 18 | (12, 26) |
| B | 15 | 156 | 11 | (7.87, 14) | |
| 2005-2006 | |||||
| A/H1N1 | 29 | 232 | 8 | (6.68, 9.59) | |
| A/H3N2 | 303 | 14 | 221 | 15 | (14, 17) |
| B | 13 | 83 | 6.5 | (5.73, 7.37) | |
14.2 Immunogenicity in Geriatric Subjects (65 years of age and older)
Tables 6 and 7 show the immunogenicity of FLUVIRIN in the geriatric age group. The six clinical studies presented enrolled a total of 296 geriatric subjects. For each of the influenza antigens, the percentage of subjects who achieved seroconversion and the percentage of subjects who achieved HI titers of ≥1:40 are shown, as well as the fold increase in GMT.
For all antigens (A/H1N1, A/H3N2 and B) at least one of the following point estimate criteria was met: the proportion of subjects with seroconversion (post-vaccination titer ≥1:40 from a pre-vaccination titer <1:10) or significant increase (at least a four-fold increase from pre-vaccination titer ≥1:10) in antibody titer was greater than 30%; the geometric mean titer (GMT) increase was >2.0; the proportion of subjects with a post-vaccination hemagglutination inhibition (HI) antibody titer ≥1:40 was greater than 60%. The pre-specified efficacy criteria were met in each study, although a relatively lower immunogenicity of A/H1N1 strain was seen in the last four studies (the same strain was in each of the formulations).
| Year/Strain | No. of subjects | Seroconversion ∞ | HI titer ≥1:40¥ | ||||
|---|---|---|---|---|---|---|---|
| N | % | 95% CIΦ | N | % | 95% CIΦ | ||
|
∞ Seroconversion: proportion of subjects with either a post-vaccination HI titer ≥1:40 from a pre-vaccination titer <1:10 or at least a four-fold increase from pre-vaccination HI titer ≥1:10 in antibody titer |
|||||||
|
¥ HI titer ≥1:40: proportion of subjects with a post-vaccination titer ≥1:40 |
|||||||
|
Φ 95% CI: 95% confidence interval |
|||||||
| 1998-1999 | |||||||
| A/H1N1 | 33 | 79 | (66, 91) | 38 | 90 | (82, 99) | |
| A/H3N2 | 42 | 33 | 79 | (66, 91) | 36 | 86 | (75, 96) |
| B | 13 | 31 | (17, 45) | 42 | 100 | (100, 100) | |
| 1999-2000 | |||||||
| A/H1N1 | 10 | 29 | (14, 45) | 23 | 68 | (52, 83) | |
| A/H3N2 | 34 | 18 | 53 | (36, 70) | 31 | 91 | (82, 100) |
| B | 9 | 26 | (12, 41) | 32 | 94 | (86, 100) | |
| 2000-2001 | |||||||
| A/H1N1 | 5 | 14 | (3, 26) | 10 | 29 | (14, 44) | |
| A/H3N2 | 35 | 22 | 63 | (47, 79) | 31 | 89 | (78, 99) |
| B | 13 | 37 | (21, 53) | 33 | 94 | (87, 100) | |
| 2001-2002 | |||||||
| A/H1N1 | 5 | 14 | (3, 26) | 14 | 40 | (24, 56) | |
| A/H3N2 | 35 | 15 | 43 | (26, 59) | 33 | 94 | (87, 100) |
| B | 6 | 17 | (5, 30) | 32 | 91 | (82, 100) | |
| 2002-2003 | |||||||
| A/H1N1 | 24 | 27 | (18, 36) | 52 | 58 | (48, 69) | |
| A/H3N2 | 89 | 42 | 47 | (37, 58) | 85 | 96 | (91, 100) |
| B | 41 | 46 | (36, 56) | 86 | 97 | (93, 100) | |
| 2004-2005 | |||||||
| A/H1N1 | 17 | 28 | (17, 41) | 46 | 75 | (63, 86) | |
| A/H3N2 | 61 | 29 | 48 | (35, 61) | 60 | 98 | (91, 100) |
| B | 38 | 62 | (49, 74) | 51 | 84 | (72, 92) | |
| Year/Strain | No. of subjects | Geometric Mean Titer (GMT) | |||
|---|---|---|---|---|---|
| Pre-vaccination | Post-vaccination | Fold Increase | (95% CI)* | ||
|
* 95% CI: 95% confidence interval |
|||||
| 1998-1999 | |||||
| A/H1N1 | 13.92 | 176.65 | 12.69 | (8.24, 19.56) | |
| A/H3N2 | 42 | 10.69 | 124.92 | 11.69 | (7.02, 19.46) |
| B | 114.1 | 273.56 | 2.40 | (1.82, 3.17) | |
| 1999-2000 | |||||
| A/H1N1 | 15.82 | 50.58 | 3.20 | (2.13, 4.80) | |
| A/H3N2 | 34 | 28.00 | 133.19 | 4.76 | (2.92, 7.76) |
| B | 57.16 | 127.86 | 2.24 | (1.56, 3.20) | |
| 2000-2001 | |||||
| A/H1N1 | 6.66 | 18.85 | 2.83 | (1.91, 4.18) | |
| A/H3N2 | 35 | 25.87 | 140.68 | 5.44 | (3.72, 7.96) |
| B | 61.24 | 191.23 | 3.12 | (2.13, 4.59) | |
| 2001-2002 | |||||
| A/H1N1 | 12.69 | 26.65 | 2.10 | (1.55, 2.84) | |
| A/H3N2 | 35 | 47.33 | 114.26 | 2.41 | (1.73, 3.38) |
| B | 45.49 | 91.89 | 2.02 | (1.47, 2.78) | |
| 2002-2003 | |||||
| A/H1N1 | 13.29 | 31.92 | 2.40 | (1.90, 3.03) | |
| A/H3N2 | 89 | 65.86 | 272.79 | 4.14 | (3.09, 5.55) |
| B | 74.87 | 288.57 | 3.85 | (2.89, 5.13) | |
| 2004-2005 | |||||
| A/H1N1 | 21 | 64 | 3.13 | (2.33, 4.2) | |
| A/H3N2 | 61 | 72 | 320 | 4.43 | (3.13, 6.27) |
| B | 20 | 114 | 5.69 | (4.39, 7.38) | |
14.3 Immunogenicity in Pediatric Subjects
A small-scale study was conducted in 1987 to evaluate safety and immunogenicity of FLUVIRIN in 38 ‘at risk’ children, with diabetes and/or asthma, or lymphoid leukemia. Thirty-eight participants aged between 4 and 12 years of age were assessed. Ten subjects had diabetes, 21 had asthma, two had both diabetes and asthma, and one had lymphoid leukemia. There were four healthy control subjects. All participants received a single 0.5-mL dose of FLUVIRIN.
Immunogenicity results were obtained for 19 of the 38 subjects enrolled in the study. The point estimate of the percentage of subjects achieving a titer of ≥ 1:40 was 84% for the A/H1N1 strain 79% for the B strain, and 53% for the A/H3N2 strain. The GMT fold increases were 5.8 for the A/H1N1 strain, 40 for the B strain and 17.7 for the A/H3N2 strain.
Three clinical studies were carried out between 1995 and 2004 in a total of 520 pediatric subjects (age range 6-47 months). Of these, 285 healthy subjects plus 41 ‘at risk’ pediatric subjects, received FLUVIRIN.
In a 1995/1996 clinical study, 41 subjects (aged 6-36 months) at increased risk for influenza-related complications received two 0.25-mL doses of FLUVIRIN. At least 49% of subjects showed a ≥4-fold increase in HI antibody titer to all three strains. HI antibody titers of 1:40 or greater were seen in at least 71% of the subjects for all three influenza strains, with increases in geometric mean titer of 6.0-fold or greater to all three strains.
Two clinical studies (1999-2000 and 2004) indicated a lower immunogenicity profile for FLUVIRIN compared with two commercial split vaccines; in a study in the age group 6-48 months the comparator was a US licensed vaccine, Fluzone®, and in another study in the age group 6-36 months the comparator was a non-US licensed inactivated influenza vaccine. Despite the small sample size (a total of 285 healthy subjects received FLUVIRIN in these two clinical studies) the lower immunogenicity profile of FLUVIRIN was greatest versus the comparator vaccines in children <36months but was also evident in those 36-48 months of age, though the differences were less.
Influenza A (H1N1) 2009 Monovalent Vaccine should only be used for the immunization of persons aged 4 years and older.
15 REFERENCES
15.1 CDC. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR 2009; 58(19): 521-4.
15.2 Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004; 103:133-138.
15.3 Hobson D, Curry RL, Beare A, et. al. The role of serum hemagglutinin-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg Camb 1972; 767-777.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
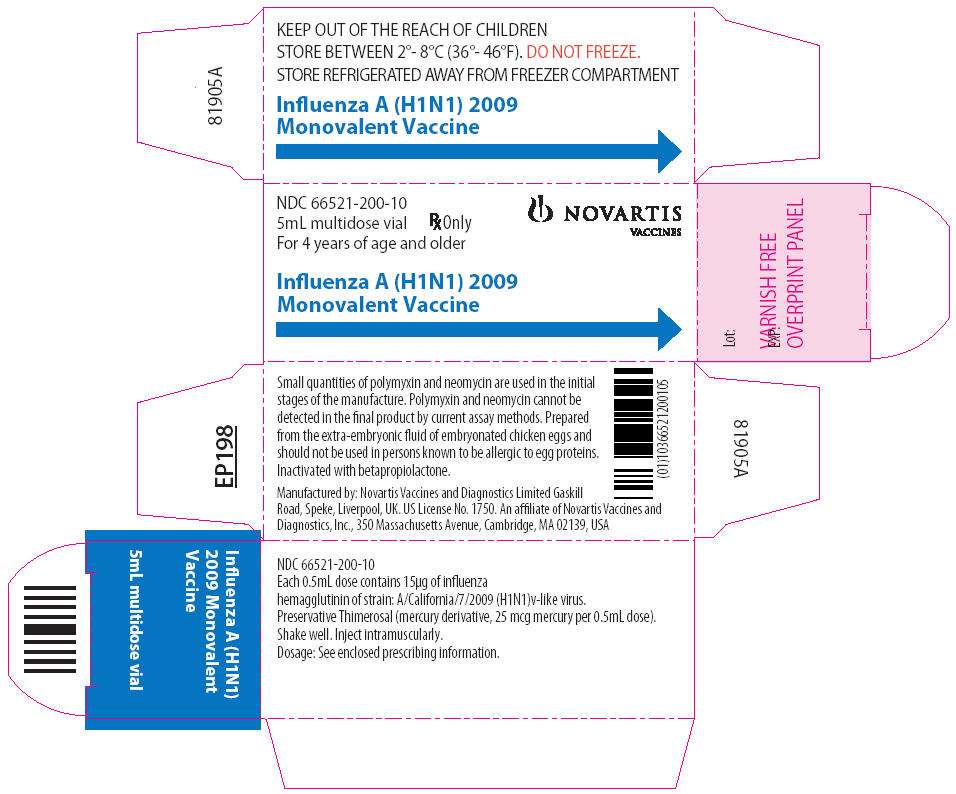
Influenza A (H1N1) 2009 Monovalent Vaccine is supplied as a 0.5-mL prefilled single dose syringe, package of 10 syringes per carton. NDC 66521-200-02
Influenza A (H1N1) 2009 Monovalent Vaccine is supplied as a 5-mL multidose vial, individually packaged in a carton. NDC 66521-200-10
16.2 Storage and Handling
Store Influenza A (H1N1) 2009 Monovalent Vaccine refrigerated between 2º and 8ºC (36º and 46ºF).
Do not freeze. Discard if the vaccine has been frozen.
Store in the original package to protect from light.
Do not use after the expiration date.
Between uses, return the multidose vial to the recommended storage conditions.
17 PATIENT COUNSELING INFORMATION
Vaccine recipients and guardians should be informed by their health care provider of the potential benefits and risks of immunization with Influenza A (H1N1) 2009 Monovalent Vaccine. When educating vaccine recipients and guardians regarding the potential side effects, clinicians should emphasize that Influenza A (H1N1) 2009 Monovalent Vaccine contains non-infectious particles and cannot cause influenza. Vaccine recipients and guardians should be instructed to report any severe or unusual adverse reactions to their healthcare provider.
Vaccine recipients should be advised that there are two influenza vaccine formulations for this influenza season, the monovalent pandemic (H1N1) 2009 influenza vaccine and seasonal trivalent influenza vaccine.
Manufactured by: Novartis Vaccines and Diagnostics Limited, Speke, Liverpool, UK
An affiliate of: Novartis Vaccines and Diagnostics, Inc., 350 Massachusetts Avenue, Cambridge, MA 02139 USA
1-800-244-7668
Package Label - Principal Display Panel - 0.5 mL Pre-Filled Syringe

Package Label - Principal Display Panel - 10 Count Carton

Package Label - Principal Display Panel - 5 mL Multi-Dose Vial

Package Label - Principal Display Panel - 1 Count Carton
Influenza A (H1N1) 2009 Monovalent VaccineInfluenza A (H1N1) 2009 Monovalent Vaccine INJECTION, SUSPENSION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||