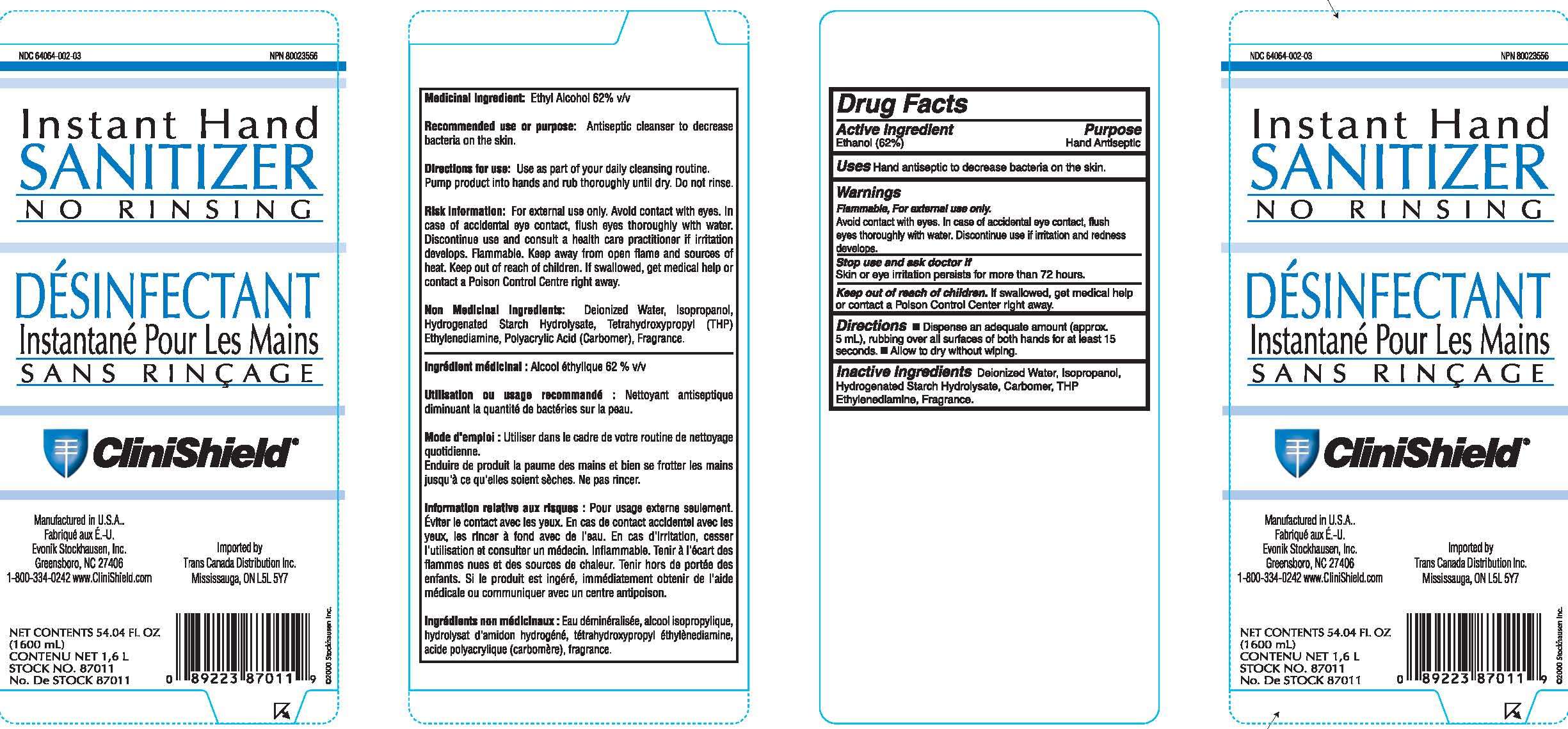
Instant Hand Sanitizer
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Instant Hand Sanitizer Uses
- Warnings
- Stop use and ask doctor if
- Keep out of reach of children.
- Directions
- Inactive Ingredients
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active Ingredient
Ethanol (62%)
Purpose
Hand Antiseptic
Instant Hand Sanitizer Uses
Hand antiseptic to decrease bacteria on the skin.
Warnings
Flammable, For external use only.
Avoid contact with eyes. In case of accidental eye contact, flush eyes thoroughly with water.
Discontinue use if irritation and redness develops.
Stop use and ask doctor if
Skin or eye irritation persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Dispense an adequate amount (approx. 5 mL), rubbing all surfaces of both hands for at least 15 seconds.
- Allow to dry without wiping.
Inactive Ingredients
Deionized Water, Isopropanol, Hydrogenated Starch Hydrolysate, Carbomer, THP Ethylendiamine, Fragrance
Principal Display Panel
NDC 64064-002-03
Instant Hand
Sanitizer
No Rinsing
Desinfectant
Instantane Pour Les Mains
Sans Rincage
Clinishield
Manufactured in U.S.A.
Fabrique Aux E.-U.
Evonik Stockhausen, LLC
Greensboro, NC 27406
1-800-334-0242 www.CliniShield.com
Imported by
Trans Canada Distribution Inc.
Mississauga, ON L5L 5Y7
1600 (mL)
Contenu Net 1.6 L
Stock No. 87011
No. De Stock 87011
Instant Hand SanitizerAlcohol LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||