Inversine
TABLETSINVERSINE(MECAMYLAMINE HCl)
FULL PRESCRIBING INFORMATION: CONTENTS*
- INVERSINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INVERSINE INDICATIONS AND USAGE
- INVERSINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INVERSINE ADVERSE REACTIONS
- OVERDOSAGE
- INVERSINE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
INVERSINE DESCRIPTION
INVERSINE®
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
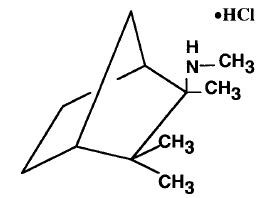
It is a white, odorless, or practically odorless, crystalline powder, is highly stable, soluble in water and has a molecular weight of 203.75.
INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
CLINICAL PHARMACOLOGY
Mecamylamine reduces blood pressure in both normotensive and hypertensive individuals. It has a gradual onset of action (1/2 to 2 hours) and a longlasting effect (usually 6 to 12 hours or more). A small oral dosage often produces a smooth and predictable reduction of blood pressure. Although this antihypertensive effect is predominantly orthostatic, the supine blood pressure is also significantly reduced.
Pharmacokinetics and Metabolism
Mecamylamine is almost completely absorbed from the gastrointestinal tract, resulting in consistent lowering of blood pressure in most patients with hypertensive cardiovascular disease. Mecamylamine is excreted slowly in the urine in the unchanged form. The rate of its renal elimination is influenced markedly by urinary pH. Alkalinization of the urine reduces, and acidification promotes, renal excretion of mecamylamine.
Mecamylamine crosses the blood-brain and placental barriers.
INVERSINE INDICATIONS AND USAGE
For the management of moderately severe to severe essential hypertension and in uncomplicated cases of malignant hypertension.
INVERSINE CONTRAINDICATIONS
INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
WARNINGS
Mecamylamine, a secondary amine, readily penetrates into the brain and thus may produce central nervous system effects. Tremor, choreiform movements, mental aberrations, and convulsions may occur rarely. These have occurred most often when large doses of INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
When ganglion blockers or other potent antihypertensive drugs are discontinued suddenly, hypertensive levels return. In patients with malignant hypertension and others, this may occur abruptly and may cause fatal cerebral vascular accidents or acute congestive heart failure. When INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
PRECAUTIONS
General
The patient's condition should be evaluated carefully, particularly as to renal and cardiovascular function. When renal, cerebral, or coronary blood flow is deficient, any additional impairment, which might result from added hypotension, must be avoided. The use of INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
The action of INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
During therapy with INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
Since urinary retention may occur in patients on ganglion blockers, caution is required in patients with prostatic hypertrophy, bladder neck obstruction, and urethral stricture.
Frequent loose bowel movements with abdominal distention and decreased borborygmi may be the first signs of paralytic ileus. If these are present, INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
Information for patients
INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
Drug Interactions
Patients receiving antibiotics and sulfonamides generally should not be treated with ganglion blockers.
The action of INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the effects upon fertility, mutagenic or carcinogenic potential of INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
Nursing Mothers
Because of the potential for serious adverse reactions in nursing infants from INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
INVERSINE ADVERSE REACTIONS
The following adverse reactions have been reported and within each category are listed in order of decreasing severity.
Gastrointestinal: Ileus, constipation (sometimes preceded by small, frequent liquid stools), vomiting, nausea, anorexia, glossitis and dryness of mouth.
Cardiovascular: Orthostatic dizziness and syncope, postural hypotension.
Nervous System/Psychiatric: Convulsions, choreiform movements, mental aberrations, tremor, and paresthesias (see WARNINGS).
Respiratory: Interstitial pulmonary edema and fibrosis.
Urogenital: Urinary retention, impotence, decreased libido.
Special Senses: Blurred vision, dilated pupils.
Miscellaneous: Weakness, fatigue, sedation.
OVERDOSAGE
Signs of overdosage include: hypotension (which may progress to peripheral vascular collapse), postural hypotension, nausea, vomiting, diarrhea, constipation, paralytic ileus, urinary retention, dizziness, anxiety, dry mouth, mydriasis, blurred vision, or palpitations. A rise in intraocular pressure may occur.
Pressor amines may be used to counteract excessive hypotension. Since patients being treated with ganglion blockers are more than normally reactive to pressor amines, small doses of the latter are recommended to avoid excessive response.
The oral LD50 of mecamylamine in the mouse is 92 mg/kg.
INVERSINE DOSAGE AND ADMINISTRATION
Therapy is usually started with one 2.5 mg tablet of INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
The average total daily dosage of INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
Administration of INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
The initial regulation of dosage should be determined by blood pressure readings in the erect position at the time of maximal effect of the drug, as well as by other signs and symptoms of orthostatic hypotension.
The effective maintenance dosage should be regulated by blood pressure readings in the erect position and by limitation of dosage to that which causes slight faintness or dizziness in this position. If the patient or a relative can use a sphygmomanometer, instructions may be given to reduce or omit a dose if readings fall below a designated level or if faintness or lightheadedness occurs. However, no change should be instituted without the knowledge of the physician.
Close supervision and education of the patient, as well as critical adjustment of dosage, are essential to successful therapy.
Other Antihypertensive Agents
When INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
HOW SUPPLIED
Tablets INVERSINE
COPYRIGHT© TARGACEPT, INC., 2002
All rights reserved
NDC 17205-0626-1 in bottles of 100.
STORAGE CONDITION
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F)
[see USP Controlled Room [Temperature]
Manufactured by: Siegfried CMS Ltd., Zofingen, Switzerland for Targacept, Inc.
Winston-Salem, NC 27101
Rev.7/02
InversineInversine TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||