Irbesartan
Macleods Pharmaceuticals Limited
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- IRBESARTAN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- IRBESARTAN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- IRBESARTAN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNING: FETAL TOXICITY
• When pregnancy is detected, discontinue irbesartan tablets as soon as possible.
• Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See WARNINGS: Fetal Toxicity.
IRBESARTAN DESCRIPTION
1
25286
CLINICAL PHARMACOLOGY
Mechanism of Action
12
112
1
Pharmacokinetics
Metabolism and Elimination
14
14
Distribution
1
Special Populations
Gender
Geriatric
max
Race
max
Renal Insufficiency
WARNINGS: Hypotension in Volume- or Salt-Depleted Patients and DOSAGE AND ADMINISTRATION
Hepatic Insufficiency
Drug Interactions
PRECAUTIONS: Drug Interactions.
Pharmacodynamics
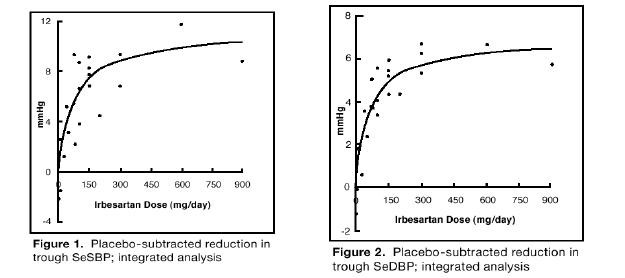
Clinical Studies
Hypertension
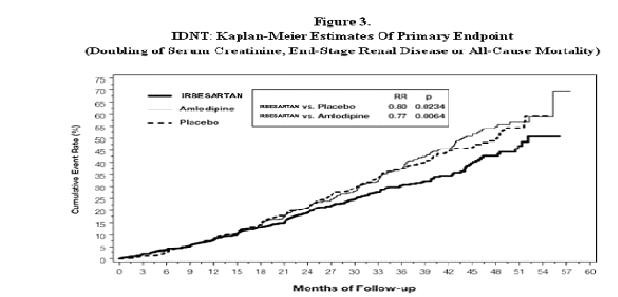
Nephropathy in Type 2 Diabetic Patients
| Irbesartan tablets N=579 (%) |
Comparison With Placebo |
Comparison With Amlodipine |
|||||
| Placebo N=569 (%) |
Hazard Ratio |
95% CI |
Amlodipine N=567 (%) |
Hazard Ratio |
95% CI |
||
| Primary Composite Endpoint |
32.6 |
39.0 |
0.80 |
0.66-0.97 (p=0.0234) |
41.1 |
0.77 |
0.63-0.93 |
| Breakdown of first occurring event contributing to primary endpoint | |||||||
| 2x creatinine | 14.2 |
19.5 |
--- |
--- |
22.8 |
--- |
--- |
| ESRD |
7.4 |
8.3 |
--- |
--- |
8.8 |
--- |
--- |
| Death |
11.1 |
11.2 |
--- |
--- |
9.5 |
--- |
--- |
| Incidence of total events over entire period of follow-up | |||||||
| 2x creatinine | 16.9 |
23.7 |
0.67 |
0.52-0.87 |
25.4 |
0.63 |
0.49-0.81 |
| ESRD | 14.2 |
17.8 |
0.77 |
0.57-1.03 |
18.3 |
0.77 |
0.57-1.03 |
| Death | 15.0 |
16.3 |
0.92 |
0.69-1.23 |
14.6 |
1.04 |
0.77-1.40 |
|
Baseline Factors |
Irbesartan tablets N=579 (%) |
Comparison With Placebo |
||
| Placebo N=569(%) |
Hazard Ratio |
95% CI |
||
| Gender | ||||
| Male | 27.5 |
36.7 |
0.68 |
0.53-0.88 |
| Female | 42.3 |
44.6 |
0.98 |
0.72-1.34 |
| Race | ||||
| White | 29.5 |
37.3 |
0.75 |
0.60-0.95 |
| Non-White | 42.6 |
43.5 |
0.95 |
0.67-1.34 |
| Age (years) | ||||
| <65 | 31.8 |
39.9 |
0.77 |
0.62-0.97 |
| ≥65 | 35.1 |
36.8 |
0.88 |
0.61-1.29 |
INDICATIONS & USAGE
Hypertension
Nephropathy in Type 2 Diabetic Patients
CLINICAL PHARMACOLOGY: Clinical Studies
IRBESARTAN CONTRAINDICATIONS
PRECAUTIONS, Drug Interactions
WARNINGS
Fetal Toxicity
Pregnancy Category D
PRECAUTIONS: Pediatric Use
Hypotension in Volume- or Salt-Depleted Patients
DOSAGE AND ADMINISTRATION
PRECAUTIONS
Impaired Renal Function
Information for Patients
Pregnancy
Drug Interactions
Non-Steroidal Anti-Inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
Dual Blockade of the Renin-Angiotensin System (RAS)
Carcinogenesis, Mutagenesis, Impairment of Fertility
0-24
0-24
Pregnancy
Pregnancy Category D
WARNINGS: Fetal Toxicity.
Nursing Mothers
Pediatric Use
Neonates with a history of in utero exposure to irbesartan
Geriatric Use
CLINICAL PHARMACOLOGY: Pharmacokinetics, Special Populations, and Clinical Studies
IRBESARTAN ADVERSE REACTIONS
Hypertension
Body as a Whole
Cardiovascular
Dermatologic
Endocrine/Metabolic/Electrolyte Imbalances
Gastrointestinal
Musculoskeletal/Connective Tissue
Nervous System
Renal/Genitourinary
Respiratory
Special Senses
Nephropathy in Type 2 Diabetic Patients
Post-Marketing Experience
Laboratory Test Findings
Hypertension
Creatinine, Blood Urea Nitrogen PRECAUTIONS: Impaired Renal Function
Hematologic:3
Nephropathy in Type 2 Diabetic Patients
Hyperkalemia
OVERDOSAGE
2
DOSAGE & ADMINISTRATION
Hypertension
CLINICAL PHARMACOLOGY: Clinical Studies
Nephropathy in Type 2 Diabetic Patients
CLINICAL PHARMACOLOGY: Clinical Studies
Volume- and Salt-Depleted Patients
WARNINGS: Hypotension in Volume- or Salt-Depleted Patients
HOW SUPPLIED
|
75 mg
|
150 mg
|
300 mg
|
|
|
Debossing |
“ ML 94” |
“ML 95” |
“ML 96” |
|
Bottle of 30 |
33342-047-07 |
33342-048-07 |
33342-049-07 |
|
Bottle of 90 |
33342-047-10 |
33342-048-10 |
33342-049-10 |
|
Bottle of 500 |
33342-047-15 |
33342-048-15 |
33342-049-15 |
|
Blister of 100 |
33342-047-12 |
33342-048-12 |
--- |
|
Blister of 90 |
--- |
--- |
33342-049-39 |
Storage
Manufactured for:
Manufactured by:
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
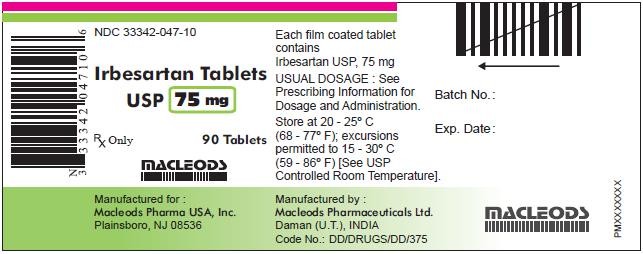

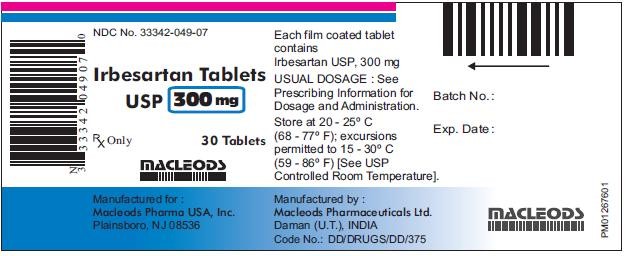

IrbesartanIrbesartan TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
IrbesartanIrbesartan TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
IrbesartanIrbesartan TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!