Irinotecan Hydrochloride
Areva Pharmaceuticals,Inc.
Areva Pharmaceuticals,Inc.
Irinotecan hydrochloride Irinotecan hydrochloride injection For Intravenous Use Only
FULL PRESCRIBING INFORMATION: CONTENTS*
- IRINOTECAN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- IRINOTECAN HYDROCHLORIDE INDICATIONS AND USAGE
- IRINOTECAN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- IRINOTECAN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- IRINOTECAN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
WARNINGS
Irinotecan hydrochloride Injection should be administered only under the supervision of a physician who is experienced in the use of cancer chemotherapeutic agents. Appropriate management of complications is possible only when adequate diagnostic and treatment facilities are readily available. Irinotecan can induce both early and late forms of diarrhea that appear to be mediated by different mechanisms. Both forms of diarrhea may be severe. Early diarrhea (occurring during or shortly after infusion of Irinotecan) may be accompanied by cholinergic symptoms of rhinitis, increased salivation, miosis, lacrimation, diaphoresis, flushing, and intestinal hyperperistalsis that can cause abdominal cramping. Early diarrhea and other cholinergic symptoms may be prevented or ameliorated by atropine (see PRECAUTIONS, General). Late diarrhea (generally occurring more than 24 hours after administration of Irinotecan) can be life threatening since it may be prolonged and may lead to dehydration, electrolyte imbalance, or sepsis. Late diarrhea should be treated promptly with loperamide. Patients with diarrhea should be carefully monitored and given fluid and electrolyte replacement if they become dehydrated or antibiotic therapy if they develop ileus, fever, or severe neutropenia (see WARNINGS). Administration of Irinotecan should be interrupted and subsequent doses reduced if severe diarrhea occurs (see DOSAGE AND ADMINISTRATION).
Severe myelosuppression may occur (see WARNINGS).
IRINOTECAN HYDROCHLORIDE DESCRIPTION
Irinotecan hydrochloride Injection is an antineoplastic agent of the topoisomerase I inhibitor class. Irinotecan hydrochloride was clinically investigated as CPT-11.
Irinotecan hydrochloride is supplied as a sterile, pale yellow, clear, aqueous solution. It is available in two single-dose sizes: 2 mL-fill vials contain 40 mg irinotecan hydrochloride and 5 mL-fill vials contain 100 mg irinotecan hydrochloride. Each milliliter of solution contains 20 mg of irinotecan hydrochloride (on the basis of the trihydrate salt), 45 mg of sorbitol NF powder, and 0.9 mg of lactic acid, USP. The pH of the solution has been adjusted to 3.5 (range, 3.2 to 3.8) with sodium hydroxide or hydrochloric acid. Irinotecan hydrochloride is intended for dilution with 5% Dextrose Injection, USP (D5W), or 0.9% Sodium Chloride Injection, USP, prior to intravenous infusion. The preferred diluent is 5% Dextrose Injection, USP.
Irinotecan hydrochloride is a semisynthetic derivative of camptothecin, an alkaloid extract from plants such as Camptotheca acuminata or is chemically synthesized.
The chemical name is (S)-4, 11-diethyl-3, 4, 12, 14-tetrahydro-4-hydroxy-3, 14-dioxo1Hpyrano [3’,4’:6,7]-indolizino [1,2-b]quinolin-9-yl-[1,4’bipiperidine]-1’-carboxylate, monohydrochloride, trihydrate. Its structural formula is as follows:

Irinotecan hydrochloride is a pale yellow to yellow crystalline powder, with the empirical formula C33H38N4O6•HCl•3H2O and a molecular weight of 677.19. It is slightly soluble in water and organic solvents.
CLINICAL PHARMACOLOGY
Irinotecan is a derivative of camptothecin. Camptothecins interact specifically with the enzyme topoisomerase I which relieves torsional strain in DNA by inducing reversible single-strand breaks. Irinotecan and its active metabolite SN-38 bind to the topoisomerase I-DNA complex and prevent religation of these single-strand breaks. Current research suggests that the cytotoxicity of irinotecan is due to double-strand DNA damage produced during DNA synthesis when replication enzymes interact with the ternary complex formed by topoisomerase I, DNA, and either irinotecan or SN-38. Mammalian cells cannot efficiently repair these double-strand breaks.
Irinotecan serves as a water-soluble precursor of the lipophilic metabolite SN-38. SN38 is formed from irinotecan by carboxylesterase-mediated cleavage of the carbamate bond between the camptothecin moiety and the dipiperidino side chain. SN-38 is approximately 1000 times as potent as irinotecan as an inhibitor of topoisomerase I purified from human and rodent tumor cell lines. In vitro cytotoxicity assays show that the potency of SN-38 relative to irinotecan varies from 2- to 2000-fold. However, the plasma area under the concentration versus time curve (AUC) values for SN-38 are 2% to 8% of irinotecan and SN-38 is 95% bound to plasma proteins compared to approximately 50% bound to plasma proteins for irinotecan (see Pharmacokinetics). The precise contribution of SN-38 to the activity of Irinotecan hydrochloride is thus unknown. Both irinotecan and SN-38 exist in an active lactone form and an inactive hydroxy acid anion form. A pH-dependent equilibrium exists between the two forms such that an acid pH promotes the formation of the lactone, while a more basic pH favors the hydroxy acid anion form.
Administration of irinotecan has resulted in antitumor activity in mice bearing cancers of rodent origin and in human carcinoma xenografts of various histological types.
Pharmacokinetics
After intravenous infusion of irinotecan in humans, irinotecan plasma concentrations decline in a multiexponential manner, with a mean terminal elimination half-life of about 6 to 12 hours. The mean terminal elimination half-life of the active metabolite SN-38 is about 10 to 20 hours. The half-lives of the lactone (active) forms of irinotecan and SN-38 are similar to those of total irinotecan and SN-38, as the lactone and hydroxy acid forms are in equilibrium.
Over the recommended dose range of 50 to 350 mg/m2, the AUC of irinotecan increases linearly with dose; the AUC of SN-38 increases less than proportionally with dose. Maximum concentrations of the active metabolite SN-38 are generally seen within 1 hour following the end of a 90-minute infusion of irinotecan. Pharmacokinetic parameters for irinotecan and SN-38 following a 90-minute infusion of irinotecan at dose levels of 125 and 340 mg/m2 determined in two clinical studies in patients with solid tumors are summarized in Table 1:
|
Cm
a
x -Maximum plasma concentration |
||||||||
|
AUC0
-
2
4
-Area under the plasma concentration-time curve from time |
||||||||
|
0 to 24 hours after the end of the 90-minute infusion |
||||||||
|
t1
/
2 - Terminal elimination half-life |
||||||||
|
Vz - Volume of distribution of terminal elimination phase |
||||||||
|
CL - Total systemic clearance |
||||||||
|
a
Plasma specimens collected for 24 hours following the end of the 90-minute infusion. |
||||||||
|
b
Plasma specimens collected for 48 hours following the end of the 90-minute infusion. Because of the longer collection period, these values provide a more accurate reflection of the terminal elimination half-lives of irinotecan and SN-38. |
||||||||
|
Dose
( mg / m2 ) |
|
|
|
Irinotecan
|
|
|
SN
-
38
|
|
|
|
Cm
a
x
(
ng
/
mL
)
|
AUC0
-
2
4
(
ng
·
h
/
mL
)
|
t1
/
2
(
h
)
|
Vz
(
L
/
m2
)
|
CL
( L / h / m2 ) |
Cm
a
x
(
ng
/
mL
)
|
AUC0
-
2
4
(
ng
·
h
/
mL
)
|
t1
/
2
(
h
)
|
| 125 (N=64) |
1,660 ± 797 |
10,200 ± 3,270 |
5.8a
±0.7 |
110 ± 48.5 |
13.3 ± 6.01 |
26.3 ± 11.9 |
229 ± 108 |
10.4a
±3.1 |
| 340 (N=6) |
3,392 ± 874 |
20,604 ± 6,027 |
11.7b
±1.0 |
234 ± 69.6 |
13.9 ± 4.0 |
56.0 ± 28.2 |
474 ± 245 |
21.0b
±4.3 |
Irinotecan exhibits moderate plasma protein binding (30% to 68% bound). SN-38 is highly bound to human plasma proteins (approximately 95% bound). The plasma protein to which irinotecan and SN-38 predominantly binds is albumin.
Metabolism and Excretion: The metabolic conversion of irinotecan to the active metabolite SN-38 is mediated by carboxylesterase enzymes and primarily occurs in the liver. In vitro studies indicate that irinotecan, SN-38 and another metabolite aminopentane carboxylic acid (APC), do not inhibit cytochrome P-450 isozymes. SN-38 is subsequently conjugated predominantly by the enzyme UDP-glucuronosyl transferase 1A1 (UGT1A1) to form a glucuronide metabolite. UGT1A1 activity is reduced in individuals with genetic polymorphisms that lead to reduced enzyme activity such as the UGT1A1*28 polymorphism. Approximately 10% of the North American population is homozygous for the UGT1A1*28 allele (also referred to as UGT1A1 7/7 genotype). In a prospective study, in which irinotecan was administered as a single-agent (350 mg/m2) on a once-every-3-week schedule, patients with the UGT1A1 7/7 genotype had a higher exposure to SN-38 than patients with the wild-type UGT1A1 allele (UGT1A1 6/6 genotype) (See WARNINGS and DOSAGE AND ADMINISTRATION). SN-38 glucuronide had 1/50 to 1/100 the activity of SN-38 in cytotoxicity assays using two cell lines in vitro. The disposition of irinotecan has not been fully elucidated in humans. The urinary excretion of irinotecan is 11% to 20%; SN-38, <1%; and SN-38 glucuronide, 3%. The cumulative biliary and urinary excretion of irinotecan and its metabolites (SN-38 and SN-38 glucuronide) over a period of 48 hours following administration of irinotecan in two patients ranged from approximately 25% (100 mg/m2) to 50% (300 mg/m2).
Pharmacokinetics in Special Populations
Geriatric
Geriatric: The pharmacokinetics of irinotecan administered using the weekly schedule was evaluated in a study of 183 patients that was prospectively designed to investigate the effect of age on irinotecan toxicity. Results from this trial indicate that there are no differences in the pharmacokinetics of irinotecan, SN-38, and SN-38 glucuronide in patients <65 years of age compared with patients ≥65 years of age. In a study of 162 patients that was not prospectively designed to investigate the effect of age, small (less than 18%) but statistically significant differences in dose-normalized irinotecan pharmacokinetic parameters in patients <65 years of age compared to patients ≥65 years of age were observed. Although dose-normalized AUC0-24 for SN-38 in patients ≥65 years of age was 11% higher than in patients <65 years of age, this difference was not statistically significant. No change in the starting dose is recommended for geriatric patients receiving the weekly dosage schedule of irinotecan. (see DOSAGE AND ADMINISTRATION).
Pediatric
See Pediatric Use under PRECAUTIONS.
Gender
The pharmacokinetics of irinotecan do not appear to be influenced by gender.
Race
The influence of race on the pharmacokinetics of irinotecan has not been evaluated.
Hepatic Insufficiency
Irinotecan clearance is diminished in patients with hepatic dysfunction while exposure to the active metabolite SN-38 is increased relative to that in patients with normal hepatic function. The magnitude of these effects is proportional to the degree of liver impairment as measured by elevations in total bilirubin and transaminase concentrations. However, the tolerability of irinotecan in patients with hepatic dysfunction (bilirubin greater than 2 mg/dl) has not been assessed sufficiently, and no recommendations for dosing can be made (see DOSAGE AND ADMINISTRATION and PRECAUTIONS: Patients at Particular Risk Sections).
Renal Insufficiency: The influence of renal insufficiency on the pharmacokinetics of irinotecan has not been evaluated. Therefore, caution should be undertaken in patients with impaired renal function. Irinotecan is not recommended for use in patients on dialysis.
Drug-Drug Interactions
5-fluorouracil (5-FU) and leucovorin (LV): In a phase 1 clinical study involving irinotecan, 5-fluorouracil (5-FU), and leucovorin (LV) in 26 patients with solid tumors, the disposition of irinotecan was not substantially altered when the drugs were coadministered. Although the Cmax and AUC0-24 of SN-38, the active metabolite, were reduced (by 14% and 8%, respectively) when irinotecan was followed by 5-FU and LV administration compared with when irinotecan was given alone, this sequence of administration was used in the combination trials and is recommended (see DOSAGE AND ADMINISTRATION). Formal in vivo or in vitro drug interaction studies to evaluate the influence of irinotecan on the disposition of 5-FU and LV have not been conducted.
Anticonvulsants: Exposure to irinotecan and its active metabolite SN-38 is substantially reduced in adult and pediatric patients concomitantly receiving the CYP3A4 enzyme-inducing anticonvulsants phenytoin, phenobarbital or Carbamazepine. The appropriate starting dose for patients taking these anticonvulsants has not been formally defined. The following drugs are also CYP3A4 inducers: rifampin, rifabutin. For patients requiring anticonvulsant treatment, consideration should be given to substituting non-enzyme inducing anticonvulsants at least 2 weeks prior to initiation of irinotecan therapy. Dexamethasone does not appear to alter the pharmacokinetics of irinotecan.
St. John’s Wort: St. John’s Wort is an inducer of CYP3A4 enzymes. Exposure to the active metabolite SN-38 is reduced in patients receiving concomitant St. John’s Wort. St. John’s Wort should be discontinued at least 2 weeks prior to the first cycle of irinotecan, and St. John’s Wort is contraindicated during irinotecan therapy.
Ketoconazole: Ketoconazole is a strong inhibitor of CYP3A4 enzymes. Patients receiving concomitant ketoconazole have increased exposure to irinotecan and its active metabolite SN-38. Patients should discontinue ketoconazole at least 1 week prior to starting irinotecan therapy and ketoconazole is contraindicated during irinotecan therapy.
Neuromuscular blocking agents. Interaction between irinotecan and neuromuscular blocking agents cannot be ruled out. Irinotecan has anticholinesterase activity, which may prolong the neuromuscular blocking effects of suxamethonium and the neuromuscular blockade of non-depolarizing drugs may be antagonized.
Atazanavir sulfate: Coadministration of atazanavir sulfate, a CYP3A4 and UGT1A1 inhibitor has the potential to increase systemic exposure to SN-38, the active metabolite of irinotecan. Physicians should take this into consideration when co-administering these drugs.
CLINICAL STUDIES
Irinotecan has been studied in clinical trials in combination with 5-fluorouracil (5-FU) and leucovorin (LV) and as a single agent (see DOSAGE AND ADMINISTRATION). When given as a component of combination-agent treatment, irinotecan was either given with a weekly schedule of bolus 5-FU/LV or with an every-2-week schedule of infusional 5-FU/LV. Weekly and a once-every-3-week dosage schedules were used for the single-agent irinotecan studies. Clinical studies of combination and single-agent use are described below.
First-Line Therapy in Combination with 5-FU/LV for the Treatment of Metastatic Colorectal Cancer
Two phase 3, randomized, controlled, multinational clinical trials support the use of Irinotecan hydrochloride Injection as first-line treatment of patients with metastatic carcinoma of the colon or rectum. In each study, combinations of irinotecan with 5-FU and LV were compared with 5-FU and LV alone. Study 1 compared combination irinotecan/bolus 5FU/ LV therapy given weekly with a standard bolus regimen of 5-FU/LV alone given daily for 5 days every 4 weeks; an irinotecan-alone treatment arm given on a weekly schedule was also included. Study 2 evaluated two different methods of administering infusional 5-FU/LV, with or without irinotecan. In both studies, concomitant medications such as antiemetics, atropine, and loperamide were given to patients for prophylaxis and/or management of symptoms from treatment. In Study 2, a 7-day course of fluoroquinolone antibiotic prophylaxis was given in patients whose diarrhea persisted for greater than 24 hours despite loperamide or if they developed a fever in addition to diarrhea. Treatment with oral fluoroquinolone was also initiated in patients who developed an absolute neutrophil count (ANC) <500/mm3, even in the absence of fever or diarrhea. Patients in both studies also received treatment with intravenous antibiotics if they had persistent diarrhea or fever or if ileus developed. In both studies, the combination of irinotecan/5-FU/LV therapy resulted in significant improvements in objective tumor response rates, time to tumor progression, and survival when compared with 5-FU/LV alone. These differences in survival were observed in spite of second-line therapy in a majority of patients on both arms, including crossover to irinotecan-containing regimens in the control arm. Patient characteristics and major efficacy results are shown in Table 2.
|
a
Study 1: N=225 (irinotecan/5-FU/LV), N=219 (5-FU/LV), N=223 (irinotecan) |
|||||
|
Study 2: N=199 (irinotecan/5-FU/LV), N=186 (5-FU/LV) |
|||||
|
bConfirmed > 4 to 6 weeks after first evidence of objective response |
|||||
|
cChi-square test |
|||||
|
dLog-rank test |
|||||
|
|
|
Study
1
|
|
Study
2
|
|
|
|
Irinotecan
+
Bolus 5 - FU / LV weekly x 4 q 6 weeks |
Bolus
5
-
FU
/
LV
daily x 5 q 4 weeks |
Irinotecan
weekly x 4 q 6 weeks |
Irinotecan
+
Infusional 5 - FU / LV |
Infusional
5 - FU / LV |
| Number of Patients |
231 |
226 |
226 |
198 |
187 |
|
Demographics
and
Treatment
Administration
|
|
|
|
|
|
| Female/Male (%) |
34/65 |
45/54 |
35/64 |
33/67 |
47/53 |
| Median Age in years (range) |
62 (25-85) |
61 (19-85) |
61 (30-87) |
62 (27-75) |
59 (24-75) |
| Performance Status (%) 0 1 2 |
39 46 15 |
41 45 13 |
46 46 8 |
51 42 7 |
51 41 8 |
| Primary Tumor (%) Colon Rectum |
81 17 |
85 14 |
84 15 |
55 45 |
65 35 |
| Median Time from Diagnosis to Randomization (months, range) |
1.9 (0-161) |
1.7 (0-203) |
1.8 (0.1-185) |
4.5 (0-88) |
2.7 (0-104) |
| Prior Adjuvant 5-FU Therapy (%) No Yes |
89 11 |
92 8 |
90 10 |
74 26 |
76 24 |
| Median Duration of Study Treatmenta (months) |
5.5 |
4.1 |
3.9 |
5.6 |
4.5 |
| Median Relative Dose Intensity (%)aIrinotecan 5-FU |
72 71 |
-- 86 |
75 -- |
87 86 |
-- 93 |
|
Efficacy
Results
|
|
|
|
|
|
| Confirmed Objective Tumor Response Rateb (%) |
39 |
21 |
18 |
35 |
22 |
|
|
|
(p < 0.0001)c
|
|
(p < 0.0005)c
|
|
| Median Time to Tumor Progressiond (months) |
7.0 |
4.3 |
4.2 |
6.7 |
4.4 |
|
|
(p=0.004)d
|
|
|
(p < 0.001)d
|
|
| Median Survival (months) |
14.8 |
12.6 |
12.0 |
17.4 |
14.1 |
|
|
(p<0.05)d
|
|
|
(p<0.05)d
|
|
Improvement was noted with irinotecan-based combination therapy relative to 5-FU/LV when response rates and time to tumor progression were examined across the following demographic and disease-related subgroups (age, gender, ethnic origin, performance status, extent of organ involvement with cancer, time from diagnosis of cancer, prior adjuvant therapy, and baseline laboratory abnormalities). Figures 1 and 2 illustrate the Kaplan-Meier survival curves for the comparison of irinotecan/5-FU/LV versus 5-FU/LV in Studies 1 and 2, respectively.
Figure 1. Survival
First-Line Irinotecan/5-FU/LV vs. 5-FU/LV
Study 1
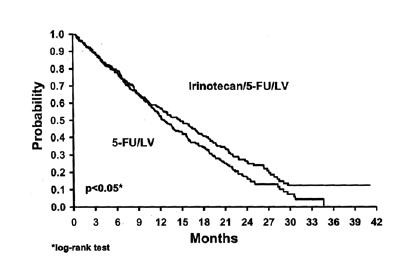
Figure 2. Survival
First-Line Irinotecan/5-FU/LV vs. 5-FU/LV
Study 2

Second-Line Treatment for Recurrent or Progressive Metastatic Colorectal Cancer After 5-FU-Based Treatment
Weekly Dosage Schedule
Data from three open-label, single-agent, clinical studies, involving a total of 304 patients in 59 centers, support the use of Irinotecan in the treatment of patients with metastatic cancer of the colon or rectum that has recurred or progressed following treatment with 5-FU-based therapy. These studies were designed to evaluate tumor response rate and do not provide information on actual clinical benefit, such as effects on survival and disease-related symptoms. In each study, Irinotecan was administered in repeated 6-week cycles consisting of a 90minute intravenous infusion once weekly for 4 weeks, followed by a 2-week rest period. Starting doses of Irinotecan in these trials were 100, 125, or 150 mg/m2, but the 150-mg/m2 dose was poorly tolerated (due to unacceptably high rates of grade 4 late diarrhea and febrile neutropenia). Study 1 enrolled 48 patients and was conducted by a single investigator at several regional hospitals. Study 2 was a multicenter study conducted by the North Central Cancer Treatment Group. All 90 patients enrolled in Study 2 received a starting dose of 125 mg/m2. Study 3 was a multicenter study that enrolled 166 patients from 30 institutions. The initial dose in Study 3 was 125 mg/m2 but was reduced to 100 mg/m2 because the toxicity seen at the 125-mg/m2 dose was perceived to be greater than that seen in previous studies. All patients in these studies had metastatic colorectal cancer, and the majority had disease that recurred or progressed following a 5-FU-based regimen administered for metastatic disease. The results of the individual studies are shown in Table 3.
|
a
Nine patients received 150 mg/m2
as a starting dose; two (22.2%) responded to irinotecan. |
||||
|
bRelative dose intensity for irinotecan based on planned dose intensity of 100, 83.3, and 66.7 mg/m2/wk corresponding with 150, 125, and 100 mg/m2
starting doses, respectively. |
||||
|
c
Confirmed ≥ 4 to 6 weeks after first evidence of objective response. |
||||
|
|
Study
|
|||
|
|
1 |
2 |
3 |
|
| Number of Patients |
48 |
90 |
64 |
102 |
| Starting Dose (mg/m2
/wk x 4) |
125a
|
125 |
125 |
100 |
|
Demographics
and
Treatment
Administration
|
||||
| Female/Male (%) |
46/54 |
36/64 |
50/50 |
51/49 |
| Median Age in years (range) |
63 (29-78) |
63 (32-81) |
61 (42-84) |
64 (25-84) |
| Ethnic Origin (%) White African American Hispanic Oriental/Asian |
79 12 8 0 |
96 4 0 0 |
81 11 8 0 |
91 5 2 2 |
| Performance Status (%) 0 1 2 |
60 38 2 |
38 48 14 |
59 33 8 |
44 51 5 |
| Primary Tumor (%) Colon Rectum Unknown |
100 0 0 |
71 29 0 |
89 11 0 |
87 8 5 |
| Prior 5-FU Therapy (%) For Metastatic Disease < 6 months after Adjuvant > 6 months after Adjuvant Classification Unknown |
81 15 2 2 |
66 7 16 12 |
73 27 0 0 |
68 28 2 3 |
| Prior Pelvic/Abdominal Irradiation (%) Yes Other None |
3 0 97 |
29 9 62 |
0 2 98 |
0 4 96 |
| Duration of Treatment with Irinotecan (median, months) |
5 |
4 |
4 |
3 |
| Relative Dose Intensityb (median %) |
74 |
67 |
73 |
81 |
|
Efficacy
|
|
|
|
|
| Confirmed Objective Response Rate (%)c(95% CI) |
21 (9.3 - 32.3) |
13 (6.3 - 20.4) |
14 (5.5 - 22.6) |
9 (3.3 - 14.3) |
| Time to Response (median, months) |
2.6 |
1.5 |
2.8 |
2.8 |
| Response Duration (median, months) |
6.4 |
5.9 |
5.6 |
6.4 |
| Survival (median, months) |
10.4 |
8.1 |
10.7 |
9.3 |
| 1-Year Survival (%) |
46 |
31 |
45 |
43 |
In the intent-to-treat analysis of the pooled data across all three studies, 193 of the 304 patients began therapy at the recommended starting dose of 125 mg/m2. Among these 193 patients, 2 complete and 27 partial responses were observed, for an overall response rate of 15.0% (95% Confidence Interval [CI], 10.0% to 20.1%) at this starting dose. A considerably lower response rate was seen with a starting dose of 100 mg/m2. The majority of responses were observed within the first two cycles of therapy, but responses did occur in later cycles of treatment (one response was observed after the eighth cycle). The median response duration for patients beginning therapy at 125 mg/m2 was 5.8 months (range, 2.6 to 15.1 months). Of the 304 patients treated in the three studies, response rates to Irinotecan were similar in males and females and among patients older and younger than 65 years. Rates were also similar in patients with cancer of the colon or cancer of the rectum and in patients with single and multiple metastatic sites. The response rate was 18.5% in patients with a performance status of 0 and 8.2% in patients with a performance status of 1 or 2. Patients with a performance status of 3 or 4 have not been studied. Over half of the patients responding to Irinotecan had not responded to prior 5-FU. Patients who had received previous irradiation to the pelvis responded to Irinotecan at approximately the same rate as those who had not previously received irradiation.
Once-Every-3-Week Dosage Schedule
Single-Arm Studies: Data from an open-label, single-agent, single-arm, multicenter, clinical study involving a total of 132 patients support a once every-3-week dosage schedule of irinotecan in the treatment of patients with metastatic cancer of the colon or rectum that recurred or progressed following treatment with 5-FU. Patients received a starting dose of 350 mg/m2 given by 30-minute intravenous infusion once every 3 weeks. Among the 132 previously treated patients in this trial, the intent-to-treat response rate was 12.1% (95% CI, 7.0% to 18.1%).
Randomized Trials: Two multicenter, randomized, clinical studies further support the use of irinotecan given by the once-every-3-week dosage schedule in patients with metastatic colorectal cancer whose disease has recurred or progressed following prior 5-FU therapy. In the first study, second-line irinotecan therapy plus best supportive care was compared with best supportive care alone. In the second study, second-line irinotecan therapy was compared with infusional 5-FU-based therapy. In both studies, irinotecan was administered intravenously at a starting dose of 350 mg/m2 over 90 minutes once every 3 weeks. The starting dose was 300 mg/m2 for patients who were 70 years and older or who had a performance status of 2. The highest total dose permitted was 700 mg. Dose reductions and/or administration delays were permitted in the event of severe hematologic and/or nonhematologic toxicities while on treatment. Best supportive care was provided to patients in both arms of Study 1 and included antibiotics, analgesics, corticosteroids, transfusions, psychotherapy, or any other symptomatic therapy as clinically indicated. In both studies, concomitant medications such as antiemetics, atropine, and loperamide were given to patients for prophylaxis and/or management of symptoms from treatment. If late diarrhea persisted for greater than 24 hours despite loperamide, a 7-day course of fluoroquinolone antibiotic prophylaxis was given. Patients in the control arm of the second study received one of the following 5-FU regimens: (1) LV, 200 mg/m2 IV over 2 hours; followed by 5-FU, 400 mg/m2 IV bolus; followed by 5-FU, 600 mg/m2 continuous IV infusion over 22 hours on days 1 and 2 every 2 weeks; (2) 5-FU, 250 to 300 mg/m2/day protracted continuous IV infusion until toxicity; (3) 5FU, 2.6 to 3 g/m2 IV over 24 hours every week for 6 weeks with or without LV, 20 to 500 mg/m2/day every week IV for 6 weeks with 2-week rest between cycles. Patients were to be followed every 3 to 6 weeks for 1 year.
A total of 535 patients were randomized in the two studies at 94 centers. The primary endpoint in both studies was survival. The studies demonstrated a significant overall survival advantage for irinotecan compared with best supportive care (p=0.0001) and infusional 5-FU-based therapy (p=0.035) as shown in Figures 3 and 4. In Study 1, median survival for patients treated with irinotecan was 9.2 months compared with 6.5 months for patients receiving best supportive care. In Study 2, median survival for patients treated with irinotecan was 10.8 months compared with 8.5 months for patients receiving infusional 5-FU-based therapy. Multiple regression analyses determined that patients’ baseline characteristics also had a significant effect on survival. When adjusted for performance status and other baseline prognostic factors, survival among patients treated with irinotecan remained significantly longer than in the control populations (p=0.001 for Study 1 and p=0.017 for Study 2). Measurements of pain, performance status, and weight loss were collected prospectively in the two studies; however, the plan for the analysis of these data was defined retrospectively. When comparing irinotecan with best supportive care in Study 1, this analysis showed a statistically significant advantage for irinotecan, with longer time to development of pain (6.9 months versus 2.0 months), time to performance status deterioration (5.7 months versus 3.3 months), and time to > 5% weight loss (6.4 months versus 4.2 months). Additionally, 33.3% (33/99) of patients with a baseline performance status of 1 or 2 showed an improvement in performance status when treated with irinotecan versus 11.3% (7/62) of patients receiving best supportive care (p=0.002). Because of the inclusion of patients with non-measurable disease, intent-to-treat response rates could not be assessed.
Figure 3. Survival
Second-Line Irinotecan vs. Best Supportive Care (BSC)
Study 1
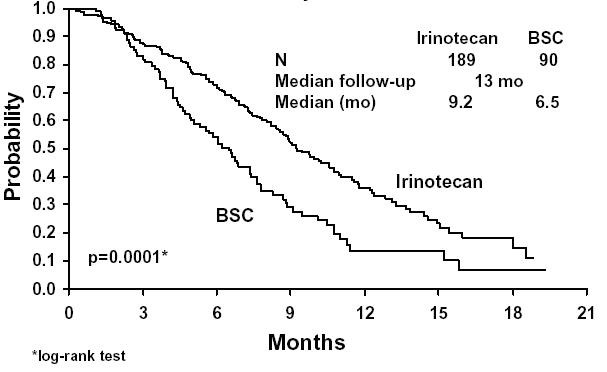
Figure 4. Survival
Second-Line Irinotecan vs. Infusional 5-FU
Study 2

In the two randomized studies, the EORTC QLQ-C30 instrument was utilized. At the start of each cycle of therapy, patients completed a questionnaire consisting of 30 questions, such as "Did pain interfere with daily activities?" (1 = Not at All, to 4 = Very Much) and "Do you have any trouble taking a long walk?" (Yes or No). The answers from the 30 questions were converted into 15 subscales, that were scored from 0 to 100, and the global health status subscale that was derived from two questions about the patient’s sense of general well being in the past week. In addition to the global health status subscale, there were five functional (i.e., cognitive, emotional, social, physical, role) and nine symptom (i.e., fatigue, appetite loss, pain assessment, insomnia, constipation, dyspnea, nausea/vomiting, financial impact, diarrhea) subscales. The results as summarized in Table 5 are based on patients’ worst post-baseline scores. In Study 1, a multivariate analysis and univariate analyses of the individual subscales were performed and corrected for multivariate testing. Patients receiving irinotecan reported significantly better results for the global health status, on two of five functional subscales, and on four of nine symptom subscales. As expected, patients receiving irinotecan noted significantly more diarrhea than those receiving best supportive care. In Study 2, the multivariate analysis on all 15 subscales did not indicate a statistically significant difference between irinotecan and infusional 5-FU.
|
a
BSC = best supportive care |
||||
|
b
Relative dose intensity for irinotecan based on planned dose intensity of 116.7 and 100 mg/m2
/wk corresponding with 350 and 300 mg/m2
starting doses, respectively. |
||||
|
|
Study
1
|
|
Study
2
|
|
|
|
Irinotecan
|
BSCa
|
Irinotecan
|
5
-
FU
|
| Number of Patients |
189 |
90 |
127 |
129 |
|
Demographics
and
Treatment
Administration
|
|
|
|
|
| Female/Male (%) |
32/68 |
42/58 |
43/57 |
35/65 |
| Median Age in years (range) |
59 (22-75) |
62 (34-75) |
58 (30-75) |
58 (25-75) |
| Performance Status (%) 0 1 2 |
47 39 14 |
31 46 23 |
58 35 8 |
54 43 3 |
| Primary Tumor (%) Colon Rectum |
55 45 |
52 48 |
57 43 |
62 38 |
| Prior 5-FU Therapy (%) For Metastatic Disease As Adjuvant Treatment |
70 30 |
63 37 |
58 42 |
68 32 |
| Prior Irradiation (%) |
26 |
27 |
18 |
20 |
| Duration of Study Treatment (median, months) (Log-rank test) |
4.1 |
-- |
4.2 (p=0.02) |
2.8 |
| Relative Dose Intensity (median %)b
|
94 |
-- |
95 |
81-99 |
|
Survival
|
|
|
|
|
| Survival (median, months) (Log-rank test) |
9.2 (p=0.0001) |
6.5 |
10.8 (p=0.035) |
8.5 |
|
aFor the five functional subscales and global health status subscale, higher scores imply better functioning, whereas, on the nine symptom subscales, higher scores imply more severe symptoms. The subscale scores of each patient were collected at each visit until the patient dropped out of the study. |
||||||
|
QLQ
-
C30
Subscale
|
|
Study
1
|
|
|
Study
2
|
|
|
|
Irinotecan
|
BSC
|
p
-
value
|
Irinotecan
|
5
-
FU
|
p
-
value
|
|
Global
Health
Status
|
47 |
37
|
0.03 |
53 |
52
|
0.9 |
|
Functional
Scales
|
|
|
|
|
|
|
| Cognitive
|
77 |
68
|
0.07 |
79 |
83
|
0.9 |
| Emotional
|
68 |
64
|
0.4 |
64 |
68
|
0.9 |
| Social
|
58 |
47
|
0.06 |
65 |
67
|
0.9 |
| Physical
|
60 |
40
|
0.0003 |
66 |
66
|
0.9 |
| Role
|
53 |
35
|
0.02 |
54 |
57
|
0.9 |
|
Symptom
Scales
|
|
|
|
|
|
|
| Fatigue
|
51 |
63
|
0.03 |
47 |
46
|
0.9 |
| Appetite Loss
|
37 |
57
|
0.0007 |
35 |
38
|
0.9 |
| Pain Assessment
|
41 |
56
|
0.009 |
38 |
34
|
0.9 |
| Insomnia
|
39 |
47
|
0.3 |
39 |
33
|
0.9 |
| Constipation
|
28 |
41
|
0.03 |
25 |
19
|
0.9 |
| Dyspnea
|
31 |
40
|
0.2 |
25 |
24
|
0.9 |
| Nausea/Vomiting
|
27 |
29
|
0.5 |
25 |
16
|
0.09 |
| Financial Impact
|
22 |
26
|
0.5 |
24 |
15
|
0.3 |
| Diarrhea
|
32 |
19
|
0.01 |
32 |
22
|
0.2 |
IRINOTECAN HYDROCHLORIDE INDICATIONS AND USAGE
Irinotecan hydrochloride Injection is indicated as a component of first-line therapy in combination with 5-fluorouracil and leucovorin for patients with metastatic carcinoma of the colon or rectum. Irinotecan is also indicated for patients with metastatic carcinoma of the colon or rectum whose disease has recurred or progressed following initial fluorouracil-based therapy.
IRINOTECAN HYDROCHLORIDE CONTRAINDICATIONS
Irinotecan hydrochloride Injection is contraindicated in patients with a known hypersensitivity to the drug or its excipients.
WARNINGS
General
Outside of a well-designed clinical study, Irinotecan hydrochloride Injection should not be used in combination with the "Mayo Clinic" regimen of 5-FU/LV (administration for 4-5 consecutive days every 4 weeks) because of reports of increased toxicity, including toxic deaths. Irinotecan should be used as recommended (see DOSAGE AND ADMINISTRATION, Table 11).
In patients receiving either irinotecan/5-FU/LV or 5-FU/LV in the clinical trials, higher rates of hospitalization, neutropenic fever, thromboembolism, first-cycle treatment discontinuation, and early deaths were observed in patients with a baseline performance status of 2 than in patients with a baseline performance status of 0 or 1.
Diarrhea
Irinotecan can induce both early and late forms of diarrhea that appear to be mediated by different mechanisms. Early diarrhea (occurring during or shortly after infusion of Irinotecan) is cholinergic in nature. It is usually transient and only infrequently is severe. It may be accompanied by symptoms of rhinitis, increased salivation, miosis, lacrimation, diaphoresis, flushing, and intestinal hyperperistalsis that can cause abdominal cramping. Early diarrhea and other cholinergic symptoms may be prevented or ameliorated by administration of atropine (see PRECAUTIONS, General, for dosing recommendations for atropine).
Late diarrhea (generally occurring more than 24 hours after administration of Irinotecan) can be life threatening since it may be prolonged and may lead to dehydration, electrolyte imbalance, or sepsis. Late diarrhea should be treated promptly with loperamide (see PRECAUTIONS, Information for Patients, for dosing recommendations for loperamide). Patients with diarrhea should be carefully monitored, should be given fluid and electrolyte replacement if they become dehydrated, and should be given antibiotic support if they develop ileus, fever, or severe neutropenia. After the first treatment, subsequent weekly chemotherapy treatments should be delayed in patients until return of pretreatment bowel function for at least 24 hours without need for anti-diarrhea medication. If grade 2, 3, or 4 late diarrhea occurs subsequent doses of Irinotecan should be decreased within the current cycle (see DOSAGE AND ADMINISTRATION).
Neutropenia
Deaths due to sepsis following severe neutropenia have been reported in patients treated with Irinotecan. Neutropenic complications should be managed promptly with antibiotic support (see PRECAUTIONS). Therapy with Irinotecan should be temporarily omitted during a cycle of therapy if neutropenic fever occurs or if the absolute neutrophil count drops <1000/mm3. After the patient recovers to an absolute neutrophil count ≥1000/mm3, subsequent doses of Irinotecan should be reduced depending upon the level of neutropenia observed (see DOSAGE AND ADMINISTRATION).
Routine administration of a colony-stimulating factor (CSF) is not necessary, but physicians may wish to consider CSF use in individual patients experiencing significant neutropenia.
Patients with Reduced UGT1A1 Activity
Individuals who are homozygous for the UGT1A1*28 allele (UGT1A1 7/7 genotype) are at increased risk for neutropenia following initiation of Irinotecan treatment.
In a study of 66 patients who received single-agent Irinotecan (350 mg/m2 once-every-3-weeks), the incidence of grade 4 neutropenia in patients homozygous for the UGT1A1*28 allele was 50%, and in patients heterozygous for this allele (UGT1A1 6/7 genotype) the incidence was 12.5%. No grade 4 neutropenia was observed in patients homozygous for the wild-type allele (UGT1A1 6/6 genotype).
In a prospective study (n=250) to investigate the role of UGT1A1*28 polymorphism in the development of toxicity in patients treated with Irinotecan (180 mg/m2) in combination with infusional 5-FU/LV, the incidence of grade 4 neutropenia in patients homozygous for the UGT1A1*28 allele was 4.5%, and in patients heterozygous for this allele the incidence was 5.3%. Grade 4 neutropenia was observed in 1.8% of patients homozygous for the wild-type allele.
In another study in which 109 patients were treated with Irinotecan (100-125 mg/m2) in combination with bolus 5-FU/LV, the incidence of grade 4 neutropenia in patients homozygous for the UGT1A1*28 allele was 18.2%, and in patients heterozygous for this allele the incidence was 11.1%. Grade 4 neutropenia was observed in 6.8% of patients homozygous for the wild-type allele.
When administered in combination with other agents, or as a single-agent, a reduction in the starting dose by at least one level of Irinotecan should be considered for patients known to be homozygous for the UGT1A1*28 allele. However, the precise dose reduction in this patient population is not known and subsequent dose modifications should be considered based on individual patient tolerance to treatment (see DOSAGE AND ADMINISTRATION and PRECAUTIONS, Laboratory Tests).
Hypersensitivity
Hypersensitivity reactions including severe anaphylactic or anaphylactoid reactions have been observed.
Colitis/Ileus
Cases of colitis complicated by ulceration, bleeding, ileus, and infection have been observed. Patients experiencing ileus should receive prompt antibiotic support (see PRECAUTIONS).
Renal Impairment/Renal Failure
Rare cases of renal impairment and acute renal failure have been identified, usually in patients who became volume depleted from severe vomiting and/or diarrhea.
Thromboembolism
Thromboembolic events have been observed in patients receiving irinotecan-containing regimens; the specific cause of these events has not been determined.
Pulmonary Toxicity
Interstitial Pulmonary Disease (IPD)-like events, including fatalities, have been reported in patients receiving irinotecan (in combination and as monotherapy) for treatment of colorectal cancer and other advanced solid tumors. In the event of an acute onset of new or progressive, unexplained pulmonary symptoms such as dyspnea, cough, and fever, irinotecan and other co-prescribed chemotherapeutic agents should be interrupted pending diagnostic evaluation. If IPD is diagnosed, irinotecan and other chemotherapy should be discontinued and appropriate treatment instituted as needed (see ADVERSE REACTIONS: Overview of Adverse Events: Respiratory).
Pregnancy
Irinotecan may cause fetal harm when administered to a pregnant woman. Radioactivity related to 14C-irinotecan crosses the placenta of rats following intravenous administration of 10 mg/kg (which in separate studies produced an irinotecan Cmax and AUC about 3 and 0.5 times, respectively, the corresponding values in patients administered 125 mg/m2). Administration of 6 mg/kg/day intravenous irinotecan to rats (which in separate studies produced an irinotecan Cmax and AUC about 2 and 0.2 times, respectively, the corresponding values in patients administered 125 mg/m2) and rabbits (about one-half the recommended human weekly starting dose on a mg/m2 basis) during the period of organogenesis, is embryotoxic as characterized by increased post-implantation loss and decreased numbers of live fetuses. Irinotecan was teratogenic in rats at doses greater than 1.2 mg/kg/day (which in separate studies produced an irinotecan Cmax and AUC about 2/3 and 1/40th, respectively, of the corresponding values in patients administered 125 mg/m2) and in rabbits at 6.0 mg/kg/day (about one-half the recommended human weekly starting dose on a mg/m2 basis). Teratogenic effects included a variety of external, visceral, and skeletal abnormalities. Irinotecan administered to rat dams for the period following organogenesis through weaning at doses of 6 mg/kg/day caused decreased learning ability and decreased female body weights in the offspring. There are no adequate and well-controlled studies of irinotecan in pregnant women. If the drug is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant while receiving treatment with Irinotecan.
PRECAUTIONS
General
Double click here to open Word Application.
Write ordered list in to word document.
Close Word Application.
Care of Intravenous Site
Irinotecan hydrochloride Injection is administered by intravenous infusion. Care should be taken to avoid extravasation, and the infusion site should be monitored for signs of inflammation. Should extravasation occur, flushing the site with sterile water and applications of ice are recommended.
Premedication with Antiemetics
Irinotecan is emetigenic. It is recommended that patients receive premedication with antiemetic agents. In clinical studies of the weekly dosage schedule, the majority of patients received 10 mg of dexamethasone given in conjunction with another type of antiemetic agent, such as a 5-HT3 blocker (e.g., ondansetron or granisetron). Antiemetic agents should be given on the day of treatment, starting at least 30 minutes before administration of Irinotecan. Physicians should also consider providing patients with an antiemetic regimen (e.g., prochlorperazine) for subsequent use as needed.
Treatment of Cholinergic Symptoms
Prophylactic or therapeutic administration of 0.25 to 1 mg of intravenous or subcutaneous atropine should be considered (unless clinically contraindicated) in patients experiencing rhinitis, increased salivation, miosis, lacrimation, diaphoresis, flushing, abdominal cramping, or diarrhea (occurring during or shortly after infusion of Irinotecan). These symptoms are expected to occur more frequently with higher irinotecan doses.
Immunosuppressant Effects/Increased Suscepibility to Infections
Administration of live or live-attenuated vaccines in patients immunocompromised by chemotherapeutic agents including Irinotecan, may result in serious or fatal infections. Avoid vaccination with a live vaccine in patients receiving irinotecan. Killed or inactivated vaccines may be administered; however, the response to such vaccines may be diminished.
Patients at Particular Risk
In patients receiving either irinotecan/5-FU/LV or 5-FU/LV in the clinical trials, higher rates of hospitalization, neutropenic fever, thromboembolism, first-cycle treatment discontinuation, and early deaths were observed in patients with a baseline performance status of 2 than in patients with a baseline performance status of 0 or 1. Patients who had previously received pelvic/abdominal radiation and elderly patients with comorbid conditions should be closely monitored.
The use of Irinotecan in patients with significant hepatic dysfunction has not been established. In clinical trials of either dosing schedule, irinotecan was not administered to patients with serum bilirubin >2.0 mg/dL, or transaminase >3 times the upper limit of normal if no liver metastasis, or transaminase >5 times the upper limit of normal with liver metastasis. In clinical trials of the weekly dosage schedule, patients with modestly elevated baseline serum total bilirubin levels (1.0 to 2.0 mg/dL) had a significantly greater likelihood of experiencing first-cycle, grade 3 or 4 neutropenia than those with bilirubin levels that were less than 1.0 mg/dL (50% [19/38] versus 18% [47/226]; p<0.001). (Also see CLINICAL PHARMACOLOGY: Pharmacokinetics in Special Populations: Hepatic Insufficiency). Patients with deficient glucuronidation of bilirubin, such as those with Gilbert’s syndrome, may be at greater risk of myelosuppression when receiving therapy with Irinotecan.
Ketoconazole, enzyme-inducing anticonvulsants and St. John’s Wort are known to have drug-drug interactions with irinotecan therapy. (See Drug-Drug Interactions sub-section under CLINICAL PHARMACOLOGY)
Irinotecan commonly causes neutropenia, leucopenia, and anemia, any of which may be severe and therefore should not be used in patients with severe bone marrow failure. Patients must not be treated with irinotecan until resolution of the bowel obstruction. Patients with hereditary fructose intolerance should not be given Irinotecan, as this product contains sorbitol.
Information for Patients
Patients and patients’ caregivers should be informed of the expected toxic effects of Irinotecan, particularly of its gastrointestinal complications, such as nausea, vomiting, abdominal cramping, diarrhea, and infection. Each patient should be instructed to have loperamide readily available and to begin treatment for late diarrhea (generally occurring more than 24 hours after administration of Irinotecan) at the first episode of poorly formed or loose stools or the earliest onset of bowel movements more frequent than normally expected for the patient. One dosage regimen for loperamide used in clinical trials consisted of the following (Note: This dosage regimen exceeds the usual dosage recommendations for loperamide.): 4 mg at the first onset of late diarrhea and then 2 mg every 2 hours until the patient is diarrhea-free for at least 12 hours. Loperamide is not recommended to be used for more than 48 consecutive hours at these doses, because of the risk of paralytic ileus. During the night, the patient may take 4 mg of loperamide every 4 hours. Premedication with loperamide is not recommended. The use of drugs with laxative properties should be avoided because of the potential for exacerbation of diarrhea. Patients should be advised to contact their physician to discuss any laxative use.
Patients should be instructed to contact their physician or nurse if any of the following occur: diarrhea for the first time during treatment; black or bloody stools; symptoms of dehydration such as lightheadedness, dizziness, or faintness; inability to take fluids by mouth due to nausea or vomiting; inability to get diarrhea under control within 24 hours; or fever or evidence of infection.
Patients should be warned about the potential for dizziness or visual disturbances which may occur within 24 hours following the administration of Irinotecan, and advised not to drive or operate machinery if these symptoms occur.
Patients should be alerted to the possibility of alopecia.
Laboratory Tests
Careful monitoring of the white blood cell count with differential, hemoglobin, and platelet count is recommended before each dose of Irinotecan.
UGT1A1 Testing
A laboratory test is available to determine the UGT1A1 status of patients. Testing can detect the UGT1A1 6/6, 6/7 and 7/7 genotypes (See WARNINGS).
Drug Interactions
The adverse effects of Irinotecan, such as myelosuppression and diarrhea, would be expected to be exacerbated by other antineoplastic agents having similar adverse effects.
Patients who have previously received pelvic/ abdominal irradiation are at increased risk of severe myelosuppression following the administration of Irinotecan. The concurrent administration of Irinotecan with irradiation has not been adequately studied and is not recommended.
Lymphocytopenia has been reported in patients receiving Irinotecan, and it is possible that the administration of dexamethasone as antiemetic prophylaxis may have enhanced the likelihood of this effect. However, serious opportunistic infections have not been observed, and no complications have specifically been attributed to lymphocytopenia.
Hyperglycemia has also been reported in patients receiving Irinotecan. Usually, this has been observed in patients with a history of diabetes mellitus or evidence of glucose intolerance prior to administration of Irinotecan. It is probable that dexamethasone, given as antiemetic prophylaxis, contributed to hyperglycemia in some patients.
The incidence of akathisia in clinical trials of the weekly dosage schedule was greater (8.5%, 4/47 patients) when prochlorperazine was administered on the same day as Irinotecan than when these drugs were given on separate days (1.3%, 1/80 patients). The 8.5% incidence of akathisia, however, is within the range reported for use of prochlorperazine when given as a premedication for other chemotherapies.
It would be expected that laxative use during therapy with Irinotecan would worsen the incidence or severity of diarrhea, but this has not been studied.
In view of the potential risk of dehydration secondary to vomiting and/or diarrhea induced by Irinotecan, the physician may wish to withhold diuretics during dosing with Irinotecan and, certainly, during periods of active vomiting or diarrhea.
Drug-Laboratory Test Interactions
There are no known interactions between Irinotecan and laboratory tests.
Carcinogenesis, Mutagenesis & Impairment of Fertility
Long-term carcinogenicity studies with irinotecan were not conducted. Rats were, however, administered intravenous doses of 2 mg/kg or 25 mg/kg irinotecan once per week for 13 weeks (in separate studies, the 25 mg/kg dose produced an irinotecan Cmax and AUC that were about 7.0 times and 1.3 times the respective values in patients administered 125 mg/m2 weekly) and were then allowed to recover for 91 weeks. Under these conditions, there was a significant linear trend with dose for the incidence of combined uterine horn endometrial stromal polyps and endometrial stromal sarcomas. Neither irinotecan nor SN-38 was mutagenic in the in vitro Ames assay. Irinotecan was clastogenic both in vitro (chromosome aberrations in Chinese hamster ovary cells) and in vivo (micronucleus test in mice). No significant adverse effects on fertility and general reproductive performance were observed after intravenous administration of irinotecan in doses of up to 6 mg/kg/day to rats and rabbits. However, atrophy of male reproductive organs was observed after multiple daily irinotecan doses both in rodents at 20 mg/kg (which in separate studies produced an irinotecan Cmax and AUC about 5 and 1 times, respectively, the corresponding values in patients administered 125 mg/m2 weekly) and dogs at 0.4 mg/kg (which in separate studies produced an irinotecan Cmax and AUC about one-half and 1/15th, respectively, the corresponding values in patients administered 125 mg/m2 weekly).
Pregnancy
Pregnancy Category D—See WARNINGS.
Nursing Mothers
Radioactivity appeared in rat milk within 5 minutes of intravenous administration of radiolabeled irinotecan and was concentrated up to 65-fold at 4 hours after administration relative to plasma concentrations. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants, it is recommended that nursing be discontinued when receiving therapy with Irinotecan.
Pediatric Use
The effectiveness of irinotecan in pediatric patients has not been established. Results from two open-label, single arm studies were evaluated. One hundred and seventy children with refractory solid tumors were enrolled in one phase 2 trial in which 50 mg/ m2 of irinotecan was infused for 5 consecutive days every 3 weeks. Grade 3-4 neutropenia was experienced by 54 (31.8%) patients. Neutropenia was complicated by fever in 15 (8.8%) patients. Grade 3-4 diarrhea was observed in 35 (20.6%) patients. This adverse event profile was comparable to that observed in adults. In the second phase 2 trial of 21 children with previously untreated rhabdomyosarcoma, 20 mg/m2 of irinotecan was infused for 5 consecutive days on weeks 0, 1, 3 and 4. This single agent therapy was followed by multimodal therapy. Accrual to the single agent irinotecan phase was halted due to the high rate (28.6%) of progressive disease and the early deaths (14%). The adverse event profile was different in this study from that observed in adults; the most significant grade 3 or 4 adverse events were dehydration experienced by 6 patients (28.6%) associated with severe hypokalemia in 5 patients (23.8%) and hyponatremia in 3 patients (14.3%); in addition Grade 3-4 infection was reported in 5 patients (23.8%) (across all courses of therapy and irrespective of causal relationship).
Pharmacokinetic parameters for irinotecan and SN-38 were determined in 2 pediatric solid-tumor trials at dose levels of 50 mg/m2 (60-min infusion, n=48) and 125 mg/m2 (90-min infusion, n=6). Irinotecan clearance (mean ± S.D.) was 17.3 ± 6.7 L/h/m2 for the 50mg/m2 dose and 16.2 ± 4.6 L/h/m2 for the 125 mg/m2 dose, which is comparable to that in adults. Dose-normalized SN-38 AUC values were comparable between adults and children. Minimal accumulation of irinotecan and SN-38 was observed in children on daily dosing regimens [daily x 5 every 3 weeks or (daily x 5) x 2 weeks every 3 weeks]. Geriatric Use Patients greater than 65 years of age should be closely monitored because of a greater risk of early and late diarrhea in this population (see CLINICAL PHARMACOLOGY, Pharmacokinetics in Special Populations and ADVERSE REACTIONS, Overview of Adverse Events). The starting dose of Irinotecan in patients 70 years and older for the once-every-3-week-dosage schedule should be 300 mg/m2 (see DOSAGE AND ADMINISTRATION).
Geriatric Use
Patients greater than 65 years of age should be closely monitored because of a greater risk of early and late diarrhea in this population (see CLINICAL PHARMACOLOGY, Pharmacokinetics in Special Populations and ADVERSE REACTIONS, Overview of Adverse Events). The starting dose of Irinotecan in patients 70 years and older for the once-every-3-week-dosage schedule should be 300 mg/m2 (see DOSAGE AND ADMINISTRATION).
IRINOTECAN HYDROCHLORIDE ADVERSE REACTIONS
First-Line Combination Therapy
A total of 955 patients with metastatic colorectal cancer received the recommended regimens of irinotecan in combination with 5-FU/LV, 5-FU/LV alone, or irinotecan alone. In the two phase 3 studies, 370 patients received irinotecan in combination with 5-FU/LV, 362 patients received 5-FU/LV alone, and 223 patients received irinotecan alone. (See Table 11 in DOSAGE AND ADMINISTRATION for recommended combination-agent regimens.)
In Study 1, 49 (7.3%) patients died within 30 days of last study treatment: 21 (9.3%) received irinotecan in combination with 5-FU/LV, 15 (6.8%) received 5-FU/LV alone, and 13 (5.8%) received irinotecan alone. Deaths potentially related to treatment occurred in 2 (0.9%) patients who received irinotecan in combination with 5-FU/LV (2 neutropenic fever/sepsis), 3 (1.4%) patients who received 5-FU/LV alone (1 neutropenic fever/sepsis, 1 CNS bleeding during thrombocytopenia, 1 unknown) and 2 (0.9%) patients who received irinotecan alone (2 neutropenic fever). Deaths from any cause within 60 days of first study treatment were reported for 15 (6.7%) patients who received irinotecan in combination with 5-FU/LV, 16 (7.3%) patients who received 5FU/ LV alone, and 15 (6.7%) patients who received irinotecan alone. Discontinuations due to adverse events were reported for 17 (7.6%) patients who received irinotecan in combination with 5FU/LV, 14 (6.4%) patients who received 5-FU/LV alone, and 26 (11.7%) patients who received irinotecan alone.
In Study 2, 10 (3.5%) patients died within 30 days of last study treatment: 6 (4.1%) received irinotecan in combination with 5-FU/LV and 4 (2.8%) received 5-FU/LV alone. There was one potentially treatment-related death, which occurred in a patient who received irinotecan in combination with 5-FU/LV (0.7%, neutropenic sepsis). Deaths from any cause within 60 days of first study treatment were reported for 3 (2.1%) patients who received irinotecan in combination with 5-FU/LV and 2 (1.4%) patients who received 5-FU/LV alone. Discontinuations due to adverse events were reported for 9 (6.2%) patients who received irinotecan in combination with 5FU/LV and 1 (0.7%) patient who received 5-FU/LV alone.
The most clinically significant adverse events for patients receiving irinotecan-based therapy were diarrhea, nausea, vomiting, neutropenia, and alopecia. The most clinically significant adverse events for patients receiving 5-FU/LV therapy were diarrhea, neutropenia, neutropenic fever, and mucositis. In Study 1, grade 4 neutropenia, neutropenic fever (defined as grade 2 fever and grade 4 neutropenia), and mucositis were observed less often with weekly irinotecan/5-FU/LV than with monthly administration of 5-FU/LV.
Tables 6 and 7 list the clinically relevant adverse events reported in Studies 1 and 2, respectively.
|
a
Severity of adverse events based on NCI CTC (version 1.0) |
||||||
|
bComplete hair loss = Grade 2 |
||||||
|
c
Includes angina pectoris, arterial thrombosis, cerebral infarct, cerebrovascular accident, deep thrombophlebitis, embolus lower extremity, heart arrest, myocardial infarct, myocardial ischemia, peripheral vascular disorder, pulmonary embolus, sudden death, thrombophlebitis, thrombosis, vascular disorder. |
||||||
|
|
Study
1
|
|||||
|
Adverse
Event
|
Irinotecan
+
Bolus 5 - FU / LV weekly x 4 q 6 weeks N = 225 |
|
Bolus 5 - FU / LV daily x 5 q 4 weeks N = 219 |
|
Irinotecan weekly x 4 q 6 weeks N = 223 |
|
|
|
Grade
1 - 4 |
Grade
3 & 4 |
Grade
1 - 4 |
Grade
3 & 4 |
Grade
1 - 4 |
Grade
3 & 4 |
| TOTAL Adverse Events |
100 |
53.3 |
100 |
45.7 |
99.6 |
45.7 |
|
GASTROINTESTINAL
Diarrhea late grade 3 grade 4 early Nausea Abdominal pain Vomiting Anorexia Constipation Mucositis |
84.9 -- -- 45.8 79.1 63.1 60.4 34.2 41.3 32.4 |
22.7 15.1 7.6 4.9 15.6 14.6 9.7 5.8 3.1 2.2 |
69.4 -- -- 31.5 67.6 50.2 46.1 42.0 31.5 76.3 |
13.2 5.9 7.3 1.4 8.2 11.5 4.1 3.7 1.8 16.9 |
83.0 -- -- 43.0 81.6 67.7 62.8 43.9 32.3 29.6 |
31.0 18.4 12.6 6.7 16.1 13.0 12.1 7.2 0.4 2.2 |
|
HEMATOLOGIC
Neutropenia grade 3 grade 4 Leukopenia Anemia Neutropenic fever Thrombocytopenia Neutropenic infection |
96.9 -- -- 96.9 96.9 -- 96.0 -- |
53.8 29.8 24.0 37.8 8.4 7.1 2.6 1.8 |
98.6 -- -- 98.6 98.6 -- 98.6 -- |
66.7 23.7 42.5 23.3 5.5 14.6 2.7 0 |
96.4 -- -- 96.4 96.9 -- 96.0 -- |
31.4 19.3 12.1 21.5 4.5 5.8 1.7 2.2 |
|
BODY
AS
A
WHOLE
Asthenia Pain Fever Infection |
70.2 30.7 42.2 22.2 |
19.5 3.1 1.7 0 |
64.4 26.9 32.4 16.0 |
11.9 3.6 3.6 1.4 |
69.1 22.9 43.5 13.9 |
13.9 2.2 0.4 0.4 |
|
METABOLIC
&
NUTRITIONAL
↑Bilirubin |
87.6 |
7.1 |
92.2 |
8.2 |
83.9 |
7.2 |
|
DERMATOLOGIC
Exfoliative dermatitis Rash Alopeciab |
0.9 19.1 43.1 |
0 0 -- |
3.2 26.5 26.5 |
0.5 0.9 -- |
0 14.3 46.1 |
0 0.4 -- |
|
RESPIRATORY
Dyspnea Cough Pneumonia |
27.6 26.7 6.2 |
6.3 1.3 2.7 |
16.0 18.3 1.4 |
0.5 0 1.0 |
22.0 20.2 3.6 |
2.2 0.4 1.3 |
|
NEUROLOGIC
Dizziness Somnolence Confusion |
23.1 12.4 7.1 |
1.3 1.8 1.8 |
16.4 4.6 4.1 |
0 1.8 0 |
21.1 9.4 2.7 |
1.8 1.3 0 |
|
CARDIOVASCULAR
Vasodilatation Hypotension Thromboembolic eventsc |
9.3 5.8 9.3 |
0.9 1.3 -- |
5.0 2.3 11.4 |
0 0.5 -- |
9.0 5.8 5.4 |
0 1.7 -- |
|
aSeverity of adverse events based on NCI CTC (version 1.0) |
||||
|
bIncludes rhinitis, increased salivation, miosis, lacrimation, diaphoresis, flushing, abdominal cramping or diarrhea (occurring during or shortly after infusion of irinotecan) |
||||
|
c
Complete hair loss = Grade 2 |
||||
|
dIncludes angina pectoris, arterial thrombosis, cerebral infarct, cerebrovascular accident, deep thrombophlebitis, embolus lower extremity, heart arrest, myocardial infarct, myocardial ischemia, peripheral vascular disorder, pulmonary embolus, sudden death, thrombophlebitis, thrombosis, vascular disorder. |
||||
|
|
Study
2
|
|
||
|
Adverse
Event
|
Irinotecan
+
5 - FU / LV infusional d 1 & 2 q 2 weeks N = 145 |
|
5 - FU / LV infusional d 1 & 2 q 2 weeks N = 143 |
|
|
|
Grade
1
-
4
|
Grade
3
&
4
|
Grade
1
-
4
|
Grade
3
&
4
|
| TOTAL Adverse Events
|
100 |
72.4 |
100 |
39.2 |
|
GASTROINTESTINAL
Diarrhea late grade 3 grade 4 Cholinergic syndromebNausea Abdominal pain Vomiting Anorexia Constipation Mucositis |
72.4 -- -- 28.3 66.9 17.2 44.8 35.2 30.3 40.0 |
14.4 10.3 4.1 1.4 2.1 2.1 3.5 2.1 0.7 4.1 |
44.8 -- -- 0.7 55.2 16.8 32.2 18.9 25.2 28.7 |
6.3 4.2 2.1 0 3.5 0.7 2.8 0.7 1.4 2.8 |
|
HEMATOLOGIC
Neutropenia grade 3 grade 4 Leukopenia Anemia Neutropenic fever Thrombocytopenia Neutropenic infection |
82.5 -- -- 81.3 97.2 -- 32.6 -- |
46.2 36.4 9.8 17.4 2.1 3.4 0 2.1 |
47.9 -- -- 42.0 90.9 -- 32.2 -- |
13.4 12.7 0.7 3.5 2.1 0.7 0 0 |
|
BODY
AS
A
WHOLE
Asthenia Pain Fever Infection |
57.9 64.1 22.1 35.9 |
9.0 9.7 0.7 7.6 |
48.3 61.5 25.9 33.6 |
4.2 8.4 0.7 3.5 |
|
METABOLIC
&
NUTRITIONAL
↑Bilirubin( |
19.1 |
3.5 |
35.9 |
10.6 |
|
DERMATOLOGIC
Hand & foot syndrome Cutaneous signs Alopeciac |
10.3 17.2 56.6 |
0.7 0.7 -- |
12.6 20.3 16.8 |
0.7 0 -- |
|
RESPIRATORY
Dyspnea |
9.7 |
1.4 |
4.9 |
0 |
|
CARDIOVASCULAR
Hypotension Thromboembolic eventsd |
3.4 11.7 |
1.4 -- |
0.7 5.6 |
0 -- |
Second-Line Single-Agent Therapy
Weekly Dosage Schedule
In three clinical studies evaluating the weekly dosage schedule, 304 patients with metastatic carcinoma of the colon or rectum that had recurred or progressed following 5FU- based therapy were treated with Irinotecan. Seventeen of the patients died within 30 days of the administration of Irinotecan; in five cases (1.6%, 5/304), the deaths were potentially drug-related. These five patients experienced a constellation of medical events that included known effects of Irinotecan. One of these patients died of neutropenic sepsis without fever. Neutropenic fever occurred in nine (3.0%) other patients; these patients recovered with supportive care.
One hundred nineteen (39.1%) of the 304 patients were hospitalized a total of 156 times because of adverse events; 81 (26.6%) patients were hospitalized for events judged to be related to administration of Irinotecan. The primary reasons for drug-related hospitalization were diarrhea, with or without nausea and/or vomiting (18.4%); neutropenia/leukopenia, with or without diarrhea and/or fever (8.2%); and nausea and/or vomiting (4.9%).
Adjustments in the dose of Irinotecan were made during the cycle of treatment and for subsequent cycles based on individual patient tolerance. The first dose of at least one cycle of Irinotecan was reduced for 67% of patients who began the studies at the 125-mg/m2 starting dose. Within-cycle dose reductions were required for 32% of the cycles initiated at the 125-mg/m2 dose level. The most common reasons for dose reduction were late diarrhea, neutropenia, and leukopenia. Thirteen (4.3%) patients discontinued treatment with Irinotecan because of adverse events. The adverse events in Table 8 are based on the experience of the 304 patients enrolled in the three studies described in the CLINICAL STUDIES, Studies Evaluating the Weekly Dosage Schedule, section.
|
a
Severity of adverse events based on NCI CTC (version 1.0) |
||
|
bOccurring > 24 hours after administration of irinotecan |
||
|
cOccurring < 24 hours after administration of irinotecan |
||
|
dPrimarily upper respiratory infections |
||
|
e
Not applicable; complete hair loss = NCI grade 2 |
||
|
|
%
of
Patients
Reporting
|
|
|
Body
System
&
Event
|
NCI
Grades
1
-
4
|
NCI
Grades
3
&
4
|
|
GASTROINTESTINAL
Diarrhea (late)b 7-9 stools/day (grade 3) > 10 stools/day (grade 4) Nausea Vomiting Anorexia Diarrhea (early)c Constipation Flatulence Stomatitis Dyspepsia |
88 - - 86 67 55 51 30 12 12 10 |
31 (16) (14) 17 12 6 8 2 0 1 0 |
|
HEMATOLOGIC
Leukopenia Anemia Neutropenia 500 to <1000/mm3 (grade 3) <500/mm3 (grade 4) |
63 60 54 - - |
28 7 26 (15) (12) |
|
BODY
AS
A
WHOLE
Asthenia Abdominal cramping/pain Fever Pain Headache Back pain Chills Minor infectiond Edema Abdominal enlargement |
76 57 45 24 17 14 14 14 10 10 |
12 16 1 2 1 2 0 0 1 0 |
|
METABOLIC
&
NUTRITIONAL
↓Body weight( Dehydration -↑Alkaline phosphatase ↑SGOT |
30 15 13 10 |
1 4 4 1 |
|
DERMATOLOGIC
Alopecia Sweating Rash |
60 16 13 |
NAe0 1 |
| RESPIRATORY Dyspnea ↑Coughing Rhinitis |
22 17 16 |
4 0 0 |
|
NEUROLOGIC
Insomnia Dizziness |
19 15 |
0 0 |
|
CARDIOVASCULAR
Vasodilation (flushing) |
11 |
0 |
Once-Every-3-Week Dosage Schedule
A total of 535 patients with metastatic colorectal cancer whose disease had recurred or progressed following prior 5-FU therapy participated in the two phase 3 studies: 316 received irinotecan, 129 received 5-FU, and 90 received best supportive care. Eleven (3.5%) patients treated with irinotecan died within 30 days of treatment. In three cases (1%, 3/316), the deaths were potentially related to irinotecan treatment and were attributed to neutropenic infection, grade 4 diarrhea, and asthenia, respectively. One (0.8%, 1/129) patient treated with 5-FU died within 30 days of treatment; this death was attributed to grade 4 diarrhea.
Hospitalizations due to serious adverse events (whether or not related to study treatment) occurred at least once in 60% (188/316) of patients who received irinotecan, 63% (57/90) who received best supportive care, and 39% (50/129) who received 5-FUbased therapy. Eight percent of patients treated with irinotecan and 7% treated with 5FU- based therapy discontinued treatment due to adverse events.
Of the 316 patients treated with irinotecan, the most clinically significant adverse events (all grades, 1-4) were diarrhea (84%), alopecia (72%), nausea (70%), vomiting (62%), cholinergic symptoms (47%), and neutropenia (30%). Table 9 lists the grade 3 and 4 adverse events reported in the patients enrolled to all treatment arms of the two studies described in the CLINICAL STUDIES, Studies Evaluating the Once-Every-3Week Dosage Schedule, section.
|
a
Severity of adverse events based on NCI CTC (version 1.0) |
||||
|
b
BSC = best supportive care |
||||
|
c
Hepatic includes events such as ascites and jaundice |
||||
|
d
Cutaneous signs include events such as rash |
||||
|
e
Respiratory includes events such as dyspnea and cough |
||||
|
f
Neurologic includes events such as somnolence |
||||
|
g
Cardiovascular includes events such as dysrhythmias, ischemia, and mechanical cardiac dysfunction |
||||
|
h
Other includes events such as accidental injury, hepatomegaly, syncope, vertigo, and weight loss |
||||
|
Adverse
Event
|
Study
1
|
|
Study
2
|
|
|
|
Irinotecan
N = 189 |
BSCb
N
=
90
|
Irinotecan
N = 127 |
5
-
FU
N = 129 |
| TOTAL Grade 3/4 Adverse Events |
79 |
67 |
69 |
54 |
|
GASTROINTESTINAL
Diarrhea Vomiting Nausea Abdominal pain Constipation Anorexia Mucositis |
22 14 14 14 10 5 2 |
6 8 3 16 8 7 1 |
22 14 11 9 8 6 2 |
11 5 4 8 6 4 5 |
|
HEMATOLOGIC
Leukopenia/Neutropenia Anemia Hemorrhage Thrombocytopenia Infection without grade 3/4 neutropenia with grade 3/4 neutropenia Fever without grade 3/4 neutropenia with grade 3/4 neutropenia |
22 7 5 1 8 1 2 2 |
0 6 3 0 3 0 1 0 |
14 6 1 4 1 2 2 4 |
2 3 3 2 4 0 0 2 |
|
BODY
AS
A
WHOLE
Pain Asthenia |
19 15 |
22 19 |
17 13 |
13 12 |
|
METABOLIC
&
NUTRITIONAL
Hepaticc |
9 |
7 |
9 |
6 |
|
DERMATOLOGIC
Hand & foot syndrome Cutaneous signsd |
0 2 |
0 0 |
0 1 |
5 3 |
|
RESPIRATORYe
|
10 |
8 |
5 |
7 |
|
NEUROLOGICf
|
12 |
13 |
9 |
4 |
|
CARDIOVASCULARg
|
9 |
3 |
4 |
2 |
|
OTHERh
|
32 |
28 |
12 |
14 |
Overview of Adverse Events
Gastrointestinal: Nausea, vomiting, and diarrhea are common adverse events following treatment with Irinotecan and can be severe. When observed, nausea and vomiting usually occur during or shortly after infusion of Irinotecan. An increased incidence of late diarrhea was observed in two studies, one using a 3-week schedule and the other using a weekly schedule. In the clinical studies testing the every 3-week-dosage schedule, the median time to the onset of late diarrhea was 5 days after irinotecan infusion. In the clinical studies evaluating the weekly dosage schedule, the median time to onset of late diarrhea was 11 days following administration of Irinotecan. For patients starting treatment at the 125-mg/m2 weekly dose, the median duration of any grade of late diarrhea was 3 days. Among those patients treated at the 125-mg/m2 weekly dose who experienced grade 3 or 4 late diarrhea, the median duration of the entire episode of diarrhea was 7 days. The frequency of grade 3 or 4 late diarrhea was somewhat greater in patients starting treatment at 125 mg/m2 than in patients given a 100-mg/m2 weekly starting dose (34% [65/193] versus 23% [24/102]; p=0.08). The frequency of grade 3 and 4 late diarrhea by age was significantly greater in patients ≥65 years than in patients <65 years (40% [53/133] versus 23% [40/171]; p=0.002). In another study of 183 patients treated on the weekly schedule, the frequency of grade 3 or 4 late diarrhea in patients ≥65 years of age was 28.6% [26/91] and in patients <65 years of age was 23.9% [22/92].
In one study of the weekly dosage treatment, the frequency of grade 3 and 4 late diarrhea was significantly greater in male than in female patients (43% [25/58] versus 16% [5/32]; p=0.01), but there were no gender differences in the frequency of grade 3 and 4 late diarrhea in the other two studies of the weekly dosage treatment schedule. Colonic ulceration, sometimes with gastrointestinal bleeding, has been observed in association with administration of Irinotecan.
Hematology: Irinotecan commonly causes neutropenia, leukopenia (including lymphocytopenia), and anemia. Serious thrombocytopenia is uncommon. When evaluated in the trials of weekly administration, the frequency of grade 3 and 4 neutropenia was significantly higher in patients who received previous pelvic/abdominal irradiation than in those who had not received such irradiation (48% [13/27] versus 24% [67/277]; p=0.04). In these same studies, patients with baseline serum total bilirubin levels of 1.0 mg/dL or more also had a significantly greater likelihood of experiencing first-cycle grade 3 or 4 neutropenia than those with bilirubin levels that were less than 1.0 mg/dL (50% [19/38] versus 18% [47/266]; p<0.001). There were no significant differences in the frequency of grade 3 and 4 neutropenia by age or gender. In the clinical studies evaluating the weekly dosage schedule, neutropenic fever (concurrent NCI grade 4 neutropenia and fever of grade 2 or greater) occurred in 3% of the patients; 6% of patients received G-CSF for the treatment of neutropenia. NCI grade 3 or 4 anemia was noted in 7% of the patients receiving weekly treatment; blood transfusions were given to 10% of the patients in these trials.
Body as a Whole: Asthenia, fever, and abdominal pain are generally the most common events of this type.
Cholinergic Symptoms: Patients may have cholinergic symptoms of rhinitis, increased salivation, miosis, lacrimation, diaphoresis, flushing, and intestinal hyperperistalsis that can cause abdominal cramping and early diarrhea. If these symptoms occur, they manifest during or shortly after drug infusion. They are thought to be related to the anticholinesterase activity of the irinotecan parent compound and are expected to occur more frequently with higher irinotecan doses.
Hepatic: In the clinical studies evaluating the weekly dosage schedule, NCI grade 3 or 4 liver enzyme abnormalities were observed in fewer than 10% of patients. These events typically occur in patients with known hepatic metastases.
Dermatologic: Alopecia has been reported during treatment with Irinotecan. Rashes have also been reported but did not result in discontinuation of treatment.
Respiratory: Severe pulmonary events are infrequent. In the clinical studies evaluating the weekly dosage schedule, NCI grade 3 or 4 dyspnea was reported in 4% of patients. Over half the patients with dyspnea had lung metastases; the extent to which malignant pulmonary involvement or other preexisting lung disease may have contributed to dyspnea in these patients is unknown.
Interstitial pulmonary disease presenting as pulmonary infiltrates is uncommon during irinotecan therapy. Interstitial pulmonary disease can be fatal. Risk factors possibly associated with the development of interstitial pulmonary disease include pre-existing lung disease, use of pneumotoxic drugs, radiation therapy, and colony stimulating factors. Patients with risk factors should be closely monitored for respiratory symptoms before and during irinotecan therapy (see WARNINGS).
Neurologic: Insomnia and dizziness can occur, but are not usually considered to be directly related to the administration of Irinotecan. Dizziness may sometimes represent symptomatic evidence of orthostatic hypotension in patients with dehydration.
Cardiovascular: Vasodilation (flushing) may occur during administration of Irinotecan. Bradycardia may also occur, but has not required intervention. These effects have been attributed to the cholinergic syndrome sometimes observed during or shortly after infusion of Irinotecan. Thromboembolic events have been observed in patients receiving Irinotecan; the specific cause of these events has not been determined.
Other Non-U.S. Clinical Trials
Irinotecan has been studied in over 1100 patients in Japan. Patients in these studies had a variety of tumor types, including cancer of the colon or rectum, and were treated with several different doses and schedules. In general, the types of toxicities observed were similar to those seen in U.S. trials with Irinotecan. There is some information from Japanese trials that patients with considerable ascites or pleural effusions were at increased risk for neutropenia or diarrhea. A potentially life-threatening pulmonary syndrome, consisting of dyspnea, fever, and a reticulonodular pattern on chest x-ray, was observed in a small percentage of patients in early Japanese studies. The contribution of irinotecan to these preliminary events was difficult to assess because these patients also had lung tumors and some had preexisting nonmalignant pulmonary disease. As a result of these observations, however, clinical studies in the United States have enrolled few patients with compromised pulmonary function, significant ascites, or pleural effusions.
Post-Marketing Experience
The following events have been identified during postmarketing use of Irinotecan in clinical practice. Myocardial ischemic events have been observed following irinotecan therapy (See also Table 7, thromboembolic events) Infrequent cases of ulcerative and ischemic colitis have been observed. This can be complicated by ulceration, bleeding, ileus, obstruction, and infection, including typhlitis. Patients experiencing ileus should receive prompt antibiotic support (see PRECAUTIONS). Cases of megacolon, intestinal perforation, symptomatic pancreatitis, and asymptomatic pancreatic enzyme elevation have been reported.
Hypersensitivity reactions including severe anaphylactic or anaphylactoid reactions have also been observed (see WARNINGS).
Cases of hyponatremia mostly related with diarrhea and vomiting have been reported. Increases in serum levels of transaminases (i.e., AST and ALT) in the absence of progressive liver metastasis; transient increase of amylase and occasionally transient increase of lipase have been reported.
Infrequent cases of renal insufficiency including acute renal failure, hypotension or circulatory failure have been observed in patients who experienced episodes of dehydration associated with diarrhea and/or vomiting, or sepsis (see WARNINGS).
Early effects such as muscular contraction or cramps and paresthesia have been reported.
Hiccups have been reported.
Transient dysarthria has been reported in patients treated with Irinotecan; in some cases, the event was attributed to the cholinergic syndrome observed during or shortly after infusion of irinotecan.
OVERDOSAGE
In U.S. phase 1 trials, single doses of up to 345 mg/m2 of irinotecan were administered to patients with various cancers. Single doses of up to 750 mg/m2 of irinotecan have been given in non-U.S. trials. The adverse events in these patients were similar to those reported with the recommended dosage and regimen. There have been reports of overdosage at doses up to approximately twice the recommended therapeutic dose, which may be fatal. The most significant adverse reactions reported were severe neutropenia and severe diarrhea. There is no known antidote for overdosage of Irinotecan. Maximum supportive care should be instituted to prevent dehydration due to diarrhea and to treat any infectious complications.
IRINOTECAN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Combination-Agent Dosage
Dosage Regimens
Irinotecan hydrochloride Injection in Combination with 5-Fluorouracil (5-FU) and Leucovorin (LV)
Irinotecan should be administered as an intravenous infusion over 90 minutes (see Preparation of Infusion Solution). For all regimens, the dose of LV should be administered immediately after Irinotecan, with the administration of 5-FU to occur immediately after receipt of LV. Irinotecan should be used as recommended; the currently recommended regimens are shown in Table 10.
|
a
Dose reductions beyond dose level –2 by decrements of ≈20% may be warranted for patients continuing to experience toxicity. Provided intolerable toxicity does not develop, treatment with additional cycles may be continued indefinitely as long as patients continue to experience clinical benefit. |
||||
|
b
Infusion follows bolus administration. |
||||
|
Regimen
1
6-wk cycle with bolus 5-FU/LV (next cycle begins on day 43) |
Irinotecan
LV 5-FU |
125mg/m2IV over90min, d 1,8,15,22 20mg/m2IV bolus, d 1,8,15,22 500mg/m2IV bolus, d 1,8,15,22 |
|
|
|
|
|
Starting
Dose
&
Modified
Dose
Levels
(
mg
/
m2
)
|
|
|
|
|
|
Starting Dose |
Dose Level-1 |
Dose Level-2 |
|
|
Irinotecan LV 5-FU |
125 20 500 |
100 20 400 |
75 20 300 |
|
Regimen
2
6-wk cycle with Infusional 5-FU/LV (next cycle begins on day 43) |
Irinotecan LV 5-FU Bolus 5-FU Infusionb |
180mg/m2IV over 90 min, d 1,15,29 200mg/m2IV over 2h, d 1,2,15,16,29,30 400mg/m2IV bolus, d 1,2,15,16,29,30 600mg/m2IV over22h, d 1,2,15,16,29,30 |
|
|
|
|
|
Starting
Dose
&
Modified
Dose
Levels
(
mg
/
m2
)
|
|
|
|
|
|
Starting Dose |
Dose Level-1 |
Dose :evel-2 |
|
|
|
180 200 400 600 |
150 200 320 480 |
120 200 240 360 |
Dosing for patients with bilirubin >2 mg/dL cannot be recommended because there is insufficient information to recommend a dose in these patients. It is recommended that patients receive premedication with antiemetic agents. Prophylactic or therapeutic administration of atropine should be considered in patients experiencing cholinergic symptoms. See PRECAUTIONS, General.
Dose Modifications
Patients should be carefully monitored for toxicity and assessed prior to each treatment. Doses of Irinotecan and 5-FU should be modified as necessary to accommodate individual patient tolerance to treatment. Based on the recommended dose-levels described in Table 10, Combination-Agent Dosage Regimens & Dose Modifications, subsequent doses should be adjusted as suggested in Table 11, Recommended Dose Modifications for Combination Schedules. All dose modifications should be based on the worst preceding toxicity. After the first treatment, patients with active diarrhea should return to pre-treatment bowel function without requiring anti-diarrhea medications for at least 24 hours before the next chemotherapy administration.
A new cycle of therapy should not begin until the toxicity has recovered to NCI grade 1 or less. Treatment may be delayed 1 to 2 weeks to allow for recovery from treatment-related toxicity. If the patient has not recovered, consideration should be given to discontinuing therapy. Provided intolerable toxicity does not develop, treatment with additional cycles of Irinotecan /5-FU/LV may be continued indefinitely as long as patients continue to experience clinical benefit.
|
aNational Cancer Institute Common Toxicity Criteria (version 1.0) |
||
|
b
Relative to the starting dose used in the previous cycle |
||
|
c
Pretreatment |
||
|
d
Excludes alopecia, anorexia, asthenia |
||
|
Patients
should
return
to
pre
-
treatment
bowel
function
without
requiring
antidiarrhea
medications
for
at
least
24
hours
before
the
next
chemotherapy
administration
.
A new cycle of therapy should not begin until the granulocyte count has recovered to > 1500 / mm3 , and the platelet count has recovered to > 100 , 000 / mm3 , and treatment - related diarrhea is fully resolved . Treatment should be delayed 1 to 2 weeks to allow for recovery from treatment - related toxicities . If the patient has not recovered after a 2 - week delay , consideration should be given to discontinuing therapy . |
||
|
Toxicity
NCI CTC gradea ( Value ) |
During
a
Cycle
of
Therapy
|
At
the
Start
of
Subsequent
Cycles of Therapyb |
| No toxicity |
Maintain dose level |
Maintain dose level |
|
Neutropenia
1 (1500 to 1999/mm3) 2 (1000 to 1499/mm3) 3 (500 to 999/mm3) 4 (<500/mm3) |
Maintain dose level ↓1 dose level Omit dose until resolved to < grade 2 then ↓ 1 dose level Omit dose until resolved to < grade 2 then ↓2, dose levels |
Maintain dose level Maintain dose level ↓1 dose level ↓2 dose levels |
|
Neutropenic
fever
|
Omit dose until resolved, then ↓ 2 dose levels |
|
|
Other
hematologic
toxicities
|
Dose modifications for leukopenia or thrombocytopenia during a cycle of therapy and at the start of subsequent cycles of therapy are also based on NCI toxicity criteria and are the same as recommended for neutropenia above. |
|
|
Diarrhea
1 (2-3 stools/day > pretxc) 2 (4-6 stools/day > pretx) 3 (7-9 stools/day > pretx) 4 (> 10 stools/day > pretx) |
Delay dose until resolved to baseline, then give same dose Omit dose until resolved to baseline, then ↓1 dose level Omit dose until resolved to baseline, then ↓1 dose level Omit dose until resolved to baseline then ↓2 dose levels |
Maintain dose level Maintain dose level ↓1 dose level ↓2 dose levels |
|
Other
nonhematologic
toxicitiesd
1 2 3 4 |
Maintain dose level Omit dose until resolved to < grade 1, then ↓1, then Omit dose until resolved to < grade 2, then ↓1 dose level Omit dose until resolved to < grade 2 then ↓2 dose level For mucositis / stomatitis decrease only 5 - FU , not irinotecan |
Maintain dose level Maintain dose level ↓1 dose level ↓2 dose levels For mucositis / stomatitis decrease only 5 - FU , not irinotecan . |
Single-Agent Dosage Schedules
Dosage Regimens
Irinotecan should be administered as an intravenous infusion over 90 minutes for both the weekly and once-every-3-week dosage schedules (see Preparation of Infusion Solution). Single agent dosage regimens are shown in Table 12.
|
a
Subsequent doses may be adjusted as high as 150 mg/m2
or to as low as 50 mg/m2
in 25 to 50 mg/m2
decrements depending upon individual patient tolerance. |
|||
|
bSubsequent doses may be adjusted as low as 200 mg/m2
in 50 mg/m2
decrements depending upon individual patient tolerance. |
|||
|
cProvided intolerable toxicity does not develop, treatment with additional cycles may be continued indefinitely as long as patients continue to experience clinical benefit. |
|||
|
Weekly
Regimena
|
125 mg/m2
IV over 90 min, d 1, 8, 15, 22 then 2-wk rest |
||
|
|
Starting
Dose
&
Modified
Dose
Levelsc
(
mg
/
m2
)
|
|
|
|
|
Starting Dose |
Dose Level -1 |
Dose Level - 2 |
|
|
125 |
100 |
75 |
|
Once
-
Every
-
3
-
Week
Regimenb
|
350 mg/m2
IV over 90 min, once every 3 wksc
|
|
|
|
|
Starting
Dose
&
Modified
Dose
Levels
(
mg
/
m2
)
|
|
|
|
|
Starting Dose |
Dose Level -1 |
Dose Level - 2 |
|
|
350 |
300 |
250 |
A reduction in the starting dose by one dose level of Irinotecan may be considered for patients with any of the following conditions: prior pelvic/abdominal radiotherapy, performance status of 2, or increased bilirubin levels. Dosing for patients with bilirubin >2 mg/dL cannot be recommended because there is insufficient information to recommend a dose in these patients.
It is recommended that patients receive premedication with antiemetic agents.
Prophylactic or therapeutic administration of atropine should be considered in patients experiencing cholinergic symptoms. See PRECAUTIONS, General.
Dose Modifications
Patients should be carefully monitored for toxicity and doses of Irinotecan should be modified as necessary to accommodate individual patient tolerance to treatment. Based on recommended dose-levels described in Table 12, Single-Agent Regimens of Irinotecan and Dose Modifications, subsequent doses should be adjusted as suggested in Table 13, Recommended Dose Modifications for Single-Agent Schedules. All dose modifications should be based on the worst preceding toxicity.
A new cycle of therapy should not begin until the toxicity has recovered to NCI grade 1 or less. Treatment may be delayed 1 to 2 weeks to allow for recovery from treatment- related toxicity. If the patient has not recovered, consideration should be given to discontinuing this combination therapy. Provided intolerable toxicity does not develop, treatment with additional cycles of Irinotecan may be continued indefinitely as long as patients continue to experience clinical benefit.
|
aAll dose modifications should be based on the worst preceding toxicity |
|||
|
bNational Cancer Institute Common Toxicity Criteria (version 1.0) |
|||
|
cPretreatment |
|||
|
dExcludes alopecia, anorexia, asthenia |
|||
|
A
new
cycle
of
therapy
should
not
begin
until
the
granulocyte
count
has
recovered
to
>
1500
/
mm3
,
and
the
platelet
count
has
recovered
to
>
100
,
000
/
mm3
,
and
treatment
-
related
diarrhea
is
fully
resolved
.
Treatment
should
be
delayed
1
to
2
weeks
to
allow
for
recovery
from
treatment
-
related
toxicities
.
If
the
patient
has
not
recovered
after
a
2
-
week
delay
,
consideration
should
be
given
to
discontinuing
irinotecan
.
|
|||
|
Worst
Toxicity
NCI Gradeb ( Value ) |
During
a
Cycle
of
Therapy
|
At
the
Start
of
the
Next
Cycles
of
Therapy
(
After
Adequate
Recovery
),
Compared
with
the
Starting
Dose
in
the
Previous
Cyclea
|
|
|
|
Weekly |
Weekly
|
Once Every 3 Weeks |
| No toxicity |
Maintain dose level |
↑25 mg/m2
up to a maximum dose of 150 mg/m2 |
Maintain dose level |
|
Neutropenia
1 (1500 to 1999/mm3) 2 (1000 to 1499/mm3) 3 (500 to 999/mm3) 4 (<500/mm3) |
Maintain dose level ↓25 mg/m2Omit dose until resolved to < grade 2, then ↓25 mg/m 2Omit dose until resolved to < grade 2, then ↓50 mg/m2 |
Maintain dose level Maintain dose level ↓25 mg/m2↓50 mg/m2 |
Maintain dose level Maintain dose level ↓50 mg/m2↓50 mg/m2 |
|
Neutropenic
fever
|
Omit dose until resolved then ↓50 mg/m 2 when resolved |
↓50 mg/m2
|
↓50 mg/m2
|
|
Other
hematologic
toxicities |
Dose modifications for leukopenia, thrombocytopenia, and anemia during a cycle of therapy and at the start of subsequent cycles of therapy are also based on NCI toxicity criteria and are the same as recommended for neutropenia above. |
|
|
|
Diarrhea
1 (2-3 stools/day > pretxc) 2 (4-6 stools/day > pretx) 3 (7-9 stools/day > pretx) 4 (> 10 stools/day > pretx) |
Maintain dose level ↓25 mg/m2Omit dose until resolved to < grade 2, then 25 mg/m2Omit dose until resolved to < grade 2 then ↓50 mg/m2 |
Maintain dose level Maintain dose level ↓25 mg/m2↓50 mg/m2 |
Maintain dose level Maintain dose level ↓50 mg/m2↓50 mg/m2 |
|
Other
nonhematologicd
Toxicities
1 2 3 4 |
Maintain dose level ↓25 mg/m2Omit dose until resolved to < grade 2, then ↓25 mg/m2Omit dose until resolved to < grade 2, then ↓50 mg/m2 |
Maintain dose level ↓25 mg/m2↓25 mg/m2↓50 mg/m2 |
Maintain dose level ↓50 mg/m2↓50 mg/m2↓50 mg/m2 |
Dosage in Patients with Reduced UGT1A1 Activity
When administered in combination with other agents, or as a single-agent, a reduction in the starting dose by at least one level of Irinotecan should be considered for patients known to be homozygous for the UGT1A1*28 allele (see CLINICAL PHARMACOLOGY and WARNINGS). However, the precise dose reduction in this patient population is not known and subsequent dose modifications should be considered based on individual patient tolerance to treatment (see Tables 10-13).
Preparation & Administration Precautions
As with other potentially toxic anticancer agents, care should be exercised in the handling and preparation of infusion solutions prepared from Irinotecan hydrochloride Injection. The use of gloves is recommended. If a solution of Irinotecan contacts the skin, wash the skin immediately and thoroughly with soap and water. If Irinotecan contacts the mucous membranes, flush thoroughly with water. Several published guidelines for handling and disposal of anticancer agents are available.1-7
Preparation of Infusion Solution
Inspect vial contents for particulate matter and repeat inspection when drug product is withdrawn from vial into syringe.
Irinotecan Injection must be diluted prior to infusion. Irinotecan should be diluted in 5% Dextrose Injection, USP, (preferred) or 0.9% Sodium Chloride Injection, USP, to a final concentration range of 0.12 to 2.8 mg/mL. In most clinical trials, Irinotecan was administered in 250 mL to 500 mL of 5% Dextrose Injection, USP.
The solution is physically and chemically stable for up to 24 hours at room temperature (approximately 25°C) and in ambient fluorescent lighting. Solutions diluted in 5% Dextrose Injection, USP, and stored at refrigerated temperatures (approximately 2° to 8°C), and protected from light are physically and chemically stable for 48 hours. Refrigeration of admixtures using 0.9% Sodium Chloride Injection, USP, is not recommended due to a low and sporadic incidence of visible particulates. Freezing Irinotecan and admixtures of Irinotecan may result in precipitation of the drug and should be avoided. Because of possible microbial contamination during dilution, it is advisable to use the admixture prepared with 5%Dextrose Injection, USP, within 24 hours if refrigerated (2° to 8°C, 36° to 46°F). In the case of admixtures prepared with 5% Dextrose Injection, USP, or Sodium Chloride Injection, USP, the solutions should be used within 6 hours if kept at room temperature (15° to 30°C, 59° to 86°F).
Other drugs should not be added to the infusion solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
HOW SUPPLIED
Each mL of Irinotecan hydrochloride Injection contains 20 mg irinotecan (on the basis of the trihydrate salt); 45 mg sorbitol; and 0.9 mg lactic acid. When necessary, pH has been adjusted to 3.5 (range, 3.2 to 3.8) with sodium hydroxide or hydrochloric acid.
Irinotecan hydrochloride Injection is available in single-dose amber glass vials in the following package sizes:
2 mL NDC 59923-702-02
5 mL NDC 59923-702-05
The vial should be inspected for damage and visible signs of leaks before removing from the carton. If damaged, incinerate the unopened package. Store at 20° to 25°C (68° to 77°F). [See USP controlled room temperature.] Protect from light and freezing. It is recommended that the vial should remain in the carton until the time of use.
Rx only
REFERENCES
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999.
- http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. ASHP guidelines on handling hazardous drugs. Am J Health-Syst Pharm. 2006; 63:1172-1193.
- Polovich, M., White, J. M., & Kelleher, L.O. (eds.) 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd. ed.) Pittsburgh, PA: Oncology Nursing Society.
Manufactured by:
Cipla Ltd.
Verna Goa, INDIA
Issued: June 2012
___________________________
i Addendum to the Expert Report on the clinical documentation, in patients with impaired hepatic function. D. Larrey, February 2001
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Vial label for Irinotecan Hydrochloride Injection 40mg/2ml
Rx only NDC 59923-702-02
IRINOTECAN HYDROCHLORIDE INJECTION
40mg/2ml
FOR INTRAVENOUS USE ONLY – must be diluted before use
CAUTION: Cytotoxic agent
2ml Single-Use Vial
Areva

Vial label for Irinotecan Hydrochloride Injection 100mg/5ml
Rx only NDC 59923-702-05
IRINOTECAN HYDROCHLORIDE INJECTION
100mg/5ml
FOR INTRAVENOUS USE ONLY – must be diluted before use
CAUTION: Cytotoxic agent
5ml Single-Use Vial
Areva

Irinotecan HydrochlorideIrinotecan INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||