Isoniazid
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- ISONIAZID DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- ISONIAZID CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- ISONIAZID ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ISONIAZID DESCRIPTION
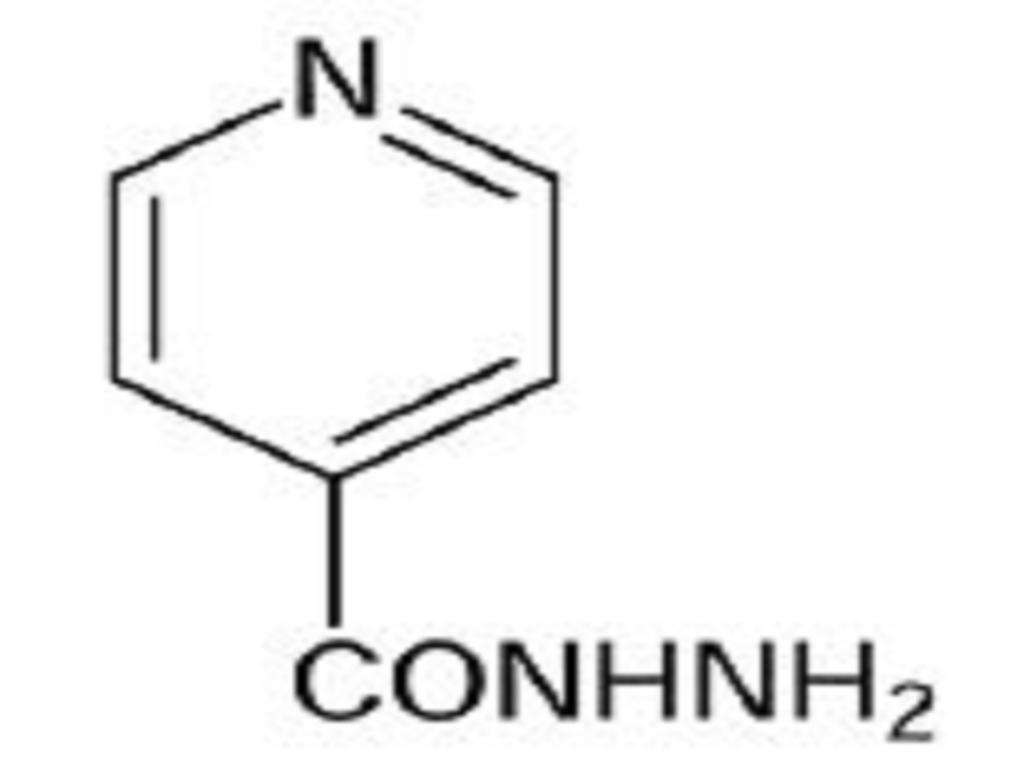
CLINICAL PHARMACOLOGY
Mechanism of Action
Microbiology
INDICATIONS & USAGE
ISONIAZID CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
GeneralLABORATORY TESTS
DRUG INTERACTIONS
FoodAcetaminophen
Carbamazepine
Ketoconazole
Phenytoin
Theophylline
Valproate
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category C
Nonteratogenic Effects
Since isoniazid is known to cross the placental barrier, neonates of isoniazid-treated mothers should be carefully observed for any evidence of adverse effects.
NURSING MOTHERS
ISONIAZID ADVERSE REACTIONS
Nervous System Reactions:
Hepatic Reactions:
Gastrointestinal Reactions:
Hematologic Reactions:
Hypersensitivity Reactions:
Metabolic And Endocrine Reactions:
Miscellaneous Reactions:
OVERDOSAGE
Signs and SymptomsTreatment
For the Asymptomatic Patient
For the Symptomatic Patient
General
Rapid Control of Metabolic Acidosis
Dialysis
DOSAGE & ADMINISTRATION
INDICATIONS AND USAGEFor Treatment of Tuberculosis
Adults
Children
Patients with Pulmonary Tuberculosis Without HIV Infection
Directly Observed Therapy (DOT)
Patients with Pulmonary Tuberculosis and HIV Infection
Patients with Extra Pulmonary Tuberculosis
Pregnant Women with Tuberculosis
Treatment of Patients with Multi-Drug Resistant Tuberculosis (MDRTB)
Directly Observed Therapy (DOT)
For Preventative Therapy of Tuberculosis
Adults over 30 Kg
Infants and Children
HOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

IsoniazidIsoniazid TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!