Isosorbide
FULL PRESCRIBING INFORMATION: CONTENTS*
- ISOSORBIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL TRIALS
- INDICATIONS & USAGE
- ISOSORBIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ISOSORBIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ISOSORBIDE DESCRIPTION
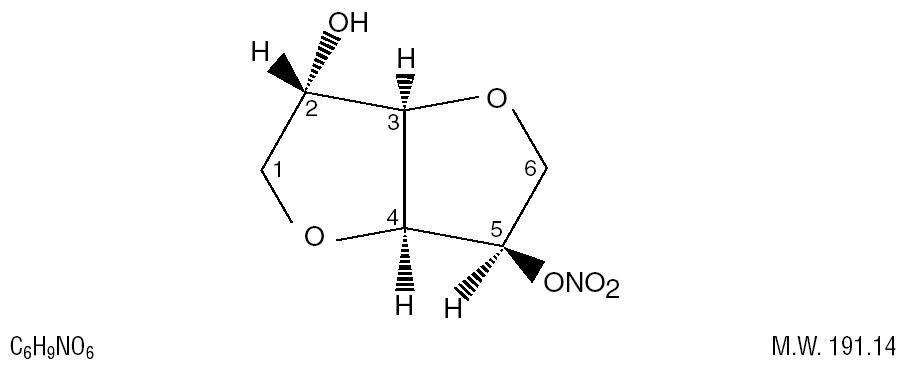
CLINICAL PHARMACOLOGY
Mechanism of ActionPharmacodynamics
Pharmacokinetics and Metabolism
SINGLE-DOSE STUDIES MULTIPLE-DOSE STUDIES ISMN Extended- ISMN Extended- ISMN Extended- PARAMETER ISMN Release Tablet Release Tablet Release Tablet 60 mg 60 mg 60mg 120 mg
Food Effects
CLINICAL TRIALS
INDICATIONS & USAGE
ISOSORBIDE CONTRAINDICATIONS
WARNINGS
Amplification of the vasodilatory effects of isosorbide mononitrate by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied. Appropriate supportive care has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the extremities and with central volume expansion.PRECAUTIONS
GeneralInformation for Patients
Drug Interactions
Drug/Laboratory Test Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy Teratogenic Effects: Pregnancy Category B
In studies designed to detect effects of isosorbide monoitrate on embryo-fetal development, doses of up to 240 or 248 mg/kg/day, administered to pregnant rats and rabbits, were unassociated with evidence of such effects. These animal doses are about 100 times the maximum recommended human dose (120 mg in a 50 kg woman) when comparison is based on body weight; when comparison is based on body surface area, the rat dose is about 17 times the human dose and the rabbit dose is about 38 times the human dose. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predicte of human response, Isosorbide Mononitrate Tablets should be used during pregnancy only if clearly needed.
Nonteratogenic Effects
Nursing Mothers
Pediatric Use
Geriatric Use
ISOSORBIDE ADVERSE REACTIONS
FREQUENCY AND ADVERSE EVENTS (DISCONTINUED)*
Three Controlled North American Studies Dose Placebo 30 mg 60 mg 120 mg** 240 mg**
Autonomic Nervous System Disorders:Dry mouth, hot flushes.
Body as a Whole:Asthenia, back pain, chest pain, edema, fatigue, fever, flu-like symptoms, malaise, rigors.
Cardiovascular Disorders, General:Cardiac failure, hypertension, hypotension.
Central and Peripheral Nervous System Disorders:Dizziness, headache, hypoesthesia, migraine, neuritis, paresis, paresthesia, ptosis, tremor, vertigo.
Gastrointestinal System Disorders:Abdominal pain, constipation, diarrhea, dyspepsia, flatulence, gastric ulcer, gastritis, glossitis, hemorrhagic gastric ulcer, hemorrhoids, loose stools, melena, nausea, vomiting.
Hearing and Vestibular Disorders:Earache, tinnitus, tympanic membrane perforation.
Heart Rate and Rhythm Disorders:Arrhythmia, arrhythmia atrial, atrial fibrillation, bradycardia, bundle branch block, extrasystole, palpitation, tachycardia, ventricular tachycardia.
Liver and Biliary System Disorders:SGOT increase, SGPT increase.
Metabolic and Nutritional Disorders:Hyperuricemia, hypokalemia.
Musculoskeletal System Disorders:Arthralgia, frozen shoulder, muscle weakness, musculoskeletal pain, myalgia, myositis, tendon disorder, torticollis.
Myo-, Endo-, Pericardial, and Valve Disorders:Angina pectoris aggravated, heart murmur, heart sound abnormal, myocardial infarction, Q wave abnormality.
Platelet, Bleeding, and Clotting Disorders:Purpura, thrombocytopenia.
Psychiatric Disorders:Anxiety, concentration impaired, confusion, decreased libido, depression, impotence, insomnia, nervousness, paroniria, somnolence.
Red Blood Cell Disorder:Hypochromic anemia.
Reproductive Disorders, Female:Atrophic vaginitis, breast pain.
Resistance Mechanism Disorders:Bacterial infection, moniliasis, viral infection.
Respiratory System Disorders:Bronchitis, bronchospasm, coughing, dyspnea, increased sputum, nasal congestion, pharyngitis, pneumonia, pulmonary infiltration, rales, rhinitis, sinusitis.
Skin and Appendages Disorders:Acne, hair texture abnormal, increased sweating, pruritus, rash, skin nodule.
Urinary System Disorders:Polyuria, renal calculus, urinary tract infection.
Vascular (Extracardiac) Disorders:Flushing, intermittent claudication, leg ulcer, varicose vein.
Vision Disorders:Conjunctivitis, photophobia, vision abnormal.
OVERDOSAGE
Hemodynamic EffectsMethemoglobinemia
DOSAGE & ADMINISTRATION
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

IsosorbideISOSORBIDE MONONITRATE TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!