Home – Isosorbide Dinitrate
Isosorbide Dinitrate
REMEDYREPACK INC.
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ISOSORBIDE DINITRATE DESCRIPTION
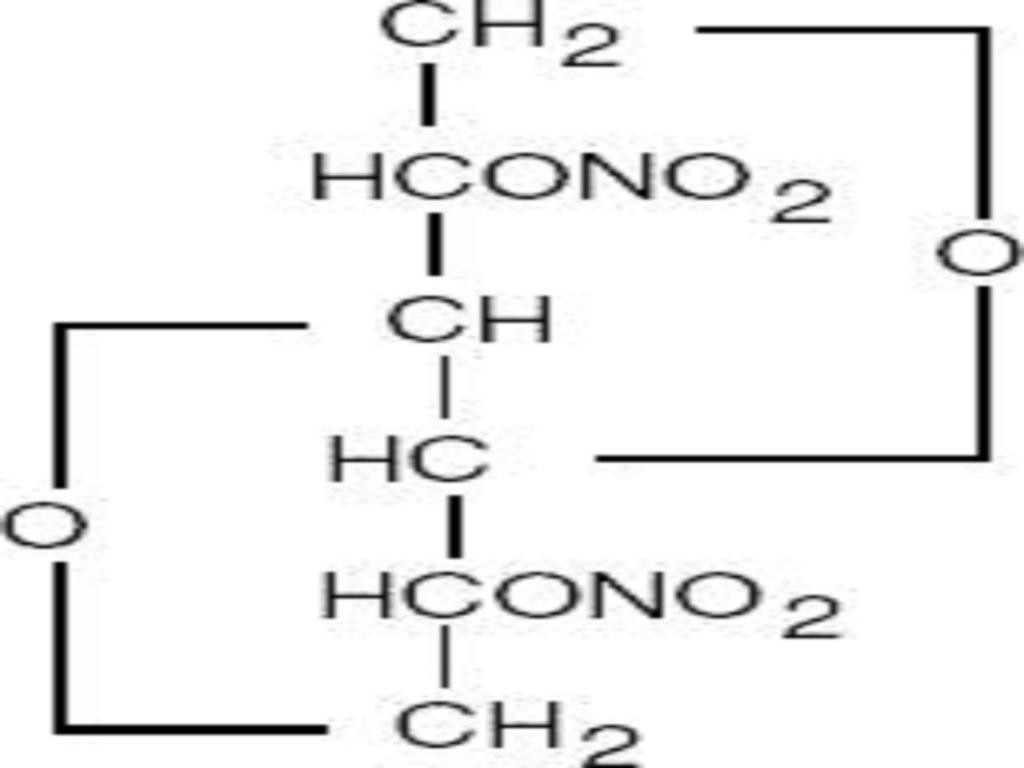
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
INDICATIONS & USAGE
WARNINGS
Amplification of the vasodilatory effects of ISDN by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied. Appropriate supportive care has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the extremities and with central volume expansion.PRECAUTIONS
ISOSORBIDE DINITRATE CONTRAINDICATIONS
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
ISOSORBIDE DINITRATE ADVERSE REACTIONS
OVERDOSAGEOVERDOSAGE
DOSAGE & ADMINISTRATION
CLINICAL PHARMACOLOGYCLINICAL PHARMACOLOGYHOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Isosorbide Dinitrate
Isosorbide Dinitrate TABLET
Product Information
|
|
Product Type
|
Human prescription drug label |
Item Code (Source)
|
NDC:49349-442(NDC:0143-1769) |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
isosorbide dinitrate ISOSORBIDE DINITRATE |
|
5 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
white |
8 mm |
westward;769 |
ROUND |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:49349-442-02 |
30 in 1 BLISTER PACK |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
ANDA |
ANDA075659 |
2011-08-01 |
|
|
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!


