ISOXSUPRINE HYDROCHLORIDE
ISOXSUPRINE HYDROCHLORIDE TABLETS, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- ISOXSUPRINE HYDROCHLORIDE DESCRIPTION
- INDICATIONS
- COMPOSITION
- ISOXSUPRINE HYDROCHLORIDE CONTRAINDICATIONS AND CAUTIONS
- ISOXSUPRINE HYDROCHLORIDE ADVERSE REACTIONS
- ISOXSUPRINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
CAUTION: Federal Law prohibits dispensing without prescription
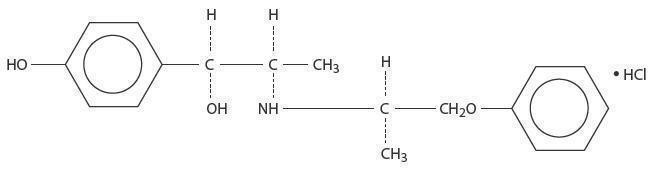
ISOXSUPRINE HYDROCHLORIDE DESCRIPTION
lsoxsuprine HCI occurs as a white odorless, crystalline powder, having a bitter
taste, It has a following structural formula
INDICATIONS
Based on a review of this drug by the National Academy of Sciences -National Research Council and / or other information, the FDA has classified the medications as follows :
Possibly Effective :
1. For the relief of symptoms associated with cerebral vascular insufficiency
2. In peripheral vascular disease of arteriosclerosis obliterans, thromboangitis obliterans (Buerger's Disease) and Raynaud's disease.
Final classification of the less than - effective indications requires further investigation.
COMPOSITION
Each tablet contains lsoxsuprine HCI 20 mg.
CONTRAINDICATIONS AND CAUTIONS
There are no known contraindications to oral use when administered in recommended doses. Should not be given immediately postpartum or in the presence of arterial bleeding.
ISOXSUPRINE HYDROCHLORIDE ADVERSE REACTIONS
On rare occasions oral administration of the drug has been associated in time with the occurrences of hypotension, tachycardia, nausea, vomiting, dizziness, abdominal distress, and severe rash. If rash appears the drug should be discontinued.
Although available evidence suggests a temporal association of these reactions with Isoxsuprine, a casual relationship can be neither confirmed nor refused.
ISOXSUPRINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
10 to 20mg three or four times daily
HOW SUPPLIED
lsoxsuprine HCI 20mg tablets are supplied in HDPE containers of 1,000's
Manufactured in India by
Vista Pharmaceuticals, Limited.

ISOXSUPRINE HYDROCHLORIDEIsoxsuprine hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||