Isradipine
Isradipine Capsules, USP
FULL PRESCRIBING INFORMATION
Isradipine is a calcium antagonist available for oral administration in capsules containing 2.5 mg or 5 mg.
The structural formula of isradipine is:
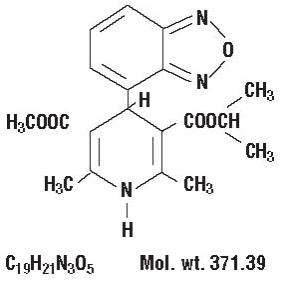
Chemically, isradipine is 3,5-Pyridinedicarboxylic acid, 4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl-, methyl 1-methylethyl ester. Isradipine is a yellow, fine crystalline powder which is odorless or has a faint characteristic odor. Isradipine is practically insoluble in water (<10 mg/L at 37ºC), but is soluble in ethanol and freely soluble in acetone, chloroform and methylene chloride.
Active Ingredient: isradipine
Inactive Ingredients: colloidal silicon dioxide, red iron oxide (2.5 mg capsule only, yellow iron oxide, gelatin, anhydrous lactose, magnesium stearate, sodium lauryl sulfate, starch (corn), titanium dioxide, black ink: black iron oxide, shellac and potassium hydroxide.
Isradipine is a dihydropyridine calcium channel blocker. It binds to calcium channels with high affinity and specificity and inhibits calcium flux into cardiac and smooth muscle. The effects observed in mechanistic experiments in vitro and studied in intact animals and man are compatible with this mechanism of action and are typical of the class.
Except for diuretic activity, the mechanism of which is not clearly understood, the pharmacodynamic effects of isradipine observed in whole animals can also be explained by calcium channel blocking activity, especially dilating effects in arterioles which reduce systemic resistance and lower blood pressure, with a small increase in resting heart rate. Although like other dihydropyridine calcium channel blockers, isradipine has negative inotropic effects in vitro, studies conducted in intact anesthetized animals have shown that the vasodilating effect occurs at doses lower than those which affect contractility. In patients with normal ventricular function, isradipine’s afterload reducing properties lead to some increase in cardiac output.
Effects in patients with impaired ventricular function have not been fully studied.
Dose-related reductions in supine and standing blood pressure are achieved within 2-3 hours following single oral doses of 2.5 mg, 5 mg, 10 mg, and 20 mg isradipine, with a duration of action (at least 50% of peak response) of more than 12 hours following administration of the highest dose.
Isradipine has been shown in controlled, double-blind clinical trials to be an effective antihypertensive agent when used as monotherapy, or when added to therapy with thiazide-type diuretics. During chronic administration, divided doses (b.i.d.) in the range of 5-20 mg daily have been shown to be effective, with response at trough (prior to next dose) over 50% of the peak blood pressure effect. The response is dose-related between 5-10 mg daily. Isradipine is equally effective in reducing supine, sitting, and standing blood pressure.
On chronic administration, increases in resting pulse rate averaged about 3-5 beats/min. these increases were not dose-related.
In man, peripheral vasodilation produced by isradipine is reflected by decreased systemic vascular resistance and increased cardiac output. Hemodynamic studies conducted in patients with normal left ventricular function produced, following intravenous isradipine administration, increases in cardiac index, stroke volume index, coronary sinus blood flow, heart rate and peak positive left ventricular dP/dt. Systemic, coronary, and pulmonary vascular resistance was decreased. These studies were conducted with doses of isradipine which produced clinically significant decreases in blood pressure. The clinical consequences of these hemodynamic effects, if any, have not been evaluated.
Effects on heart rate are variable, dependent upon rate of administration and presence of underlying cardiac condition. While increases in both peak positive dP/dt and LV ejection fraction are seen when intravenous isradipine is given, it is impossible to conclude that these represent a positive inotropic effect due to simultaneous changes in preload and afterload. In patients with coronary artery disease undergoing atrial pacing during cardiac catheterization, intravenous isradipine diminished abnormalities of systolic performance. In patients with moderate left ventricular dysfunction, oral and intravenous isradipine in doses which reduce blood pressure by 12% to 30%, resulted in improvement in cardiac index without increase in heart rate, and with no change or reduction in pulmonary capillary wedge pressure. Combination of isradipine and propranolol did not significantly affect left ventricular dP/dt max. The clinical consequences of these effects have not been evaluated.
In general, no detrimental effects on the cardiac conduction system were seen with the use of isradipine. Electrophysiologic studies were conducted on patients with normal sinus and atrioventricular node function. Intravenous isradipine in doses which reduce systolic blood pressure did not affect PR, QRS, AH* or HV* intervals.
No changes were seen in Wenckebach cycle length, atrial, and ventricular refractory periods. Slight prolongation of QTc interval of 3% was seen in one study. Effects on sinus node recovery time (CSNRT) were mild or not seen.
In patients with sick sinus syndrome, at doses which significantly reduced blood pressure, intravenous isradipine resulted in no depressant effect on sinus and atrioventricular node function.
*AH = conduction time from low right atrium to His bundle deflection, or AV nodal conduction time; HV = conduction time through His bundle and the bundle branch-Purkinje system.
Isradipine is 90%-95% absorbed and is subject to extensive first-pass metabolism, resulting in a bioavailability of about 15%-24%. Isradipine is detectable in plasma within 20 minutes after administration of single oral doses of 2.5-20 mg, and peak concentrations of approximately 1 ng/mL/mg dosed occur about 1.5 hours after drug administration. Administration of isradipine with food significantly increases the time to peak by about an hour, but has no effect on the total bioavailability (area under the curve) of the drug. Isradipine is 95% bound to plasma proteins. Both peak plasma concentration and AUC exhibit a linear relationship to dose over the 0-20 mg dose range. The elimination of isradipine is biphasic with an early half-life of 1 ½-2 hours, and a terminal half-life of about 8 hours. The total body clearance of isradipine is 1.4 L/min and the apparent volume od disturbance is 3 L/kg.
Isradipine is completely metabolized prior to excretion, and no unchanged drug is detected in the urine. Six metabolites have been characterized in blood and urine, with the mono acids of the pyridine derivative and a cyclic lactone product accounting for >75% fo the material identified. Approximately 60%-65% of an administered dose is excreted in the urine and 25%-30% in the feces. Mild renal impairment (creatinine clearance 30 to 80 mL/min) increases the bioavailability (AUC) of isradipine by 45%. Progressive deterioration reverses this trend, and patients with severe renal failure (creatinine clearance <10 mL/min) who have been on hemodialysis show a 20% to 50% lower AUC than healthy volunteers. No pharmacokinetic information is available in drug therapy during hemodialysis. In elderly patients Cmax and AUC are increased by 13% and 40%, respectively; in patients with hepatic impairment, Cmax and AUC are increased by 32% and 52%, respectively (see DOSAGE AND ADMINISTRATION ).
Isradipine is indicated in the management of hypertension. It may be used alone or concurrently with thiazide-type diuretics.
Isradipine is contraindicated in individuals who have shown hypersensitivity to any of the ingredients in the formulation.
None
Blood Pressure: Because isradipine decreases peripheral resistance, like other calcium blockers isradipine may occasionally produce symptomatic hypotension. However, symptoms like syncope and severe dizziness have rarely been reported in hypertensive patients administered isradipine, particularly at the initial recommended doses (see DOSAGE AND ADMINISTRATION ).
Use in Patients with Congestive Heart Failure: Although acute hemodynamic studies in patients with congestive heart failure have shown that isradipine reduced afterload without impairing myocardial contractility, it has a negative inotropic effect at high doses in vitro and possibly in some patients. Caution should be exercised when using isradipine in congestive heart failure patients, particularly in combination with a beta-blocker.
Nitroglycerin: Isradipine has been safely coadministered with nitroglycerin.
Hydrochlorothiazide: A study in normal healthy volunteers has shown that concomitant administration of isradipine and hydrochlorothiazide does not result in altered pharmacokinetics of either drug. In a study in hypertensive patients, addition of isradipine to existing hydrochlorothiazide therapy did not result in any unexpected adverse effects, and isradipine had an additional antihypertensive effect.
Propranolol: In a single dose study in normal volunteers, co-administration of propranolol had a small effect on the rate but no effect on the extent of isradipine bioavailability. Significant increases in AUC (27%) and Cmax (58%) and decreases in tmax (23%) of propranolol were noted in this study. However, concomitant administration of 5 mg b.i.d. isradipine and 40 mg b.i.d. propranolol to healthy volunteers under steady-state conditions had no relevant effect on either drug’s bioavailability. AUC and Cmax differences were <20% between isradipine given singly and in combination with propranolol, and between propranolol given singly and in combination with isradipine.
Cimetidine: In a study in healthy volunteers, a one-week course of cimetidine at 400 mg b.i.d. with a single 5 mg dose of isradipine on the sixth day showed an increase in isradipine mean peak plasma concentrations (36%) and significant increase in area under the curve (50%). If isradipine therapy is initiated in a patient currently receiving cimetidine, careful monitoring for adverse reactions is advised and downward dose adjustment may be required.
Rifampicin: In a study with healthy volunteers, a six-day course of rifampicin at 600 mg/day followed by a single 5 mg dose of isradipine resulted in a reduction in isradipine levels to below detectable limits. If rifampicin therapy is required, isradipine concentrations and therapeutic effects are likely to be markedly reduced or abolished as a consequence of increased metabolism and higher clearance of isradipine.
Warfarin: In a study with healthy volunteers, no clinically relevant pharmacokinetic or pharmacodynamic interaction between isradipine and racemic warfarin was seen when two single oral doses of warfarin (0.7 mg/kg body weight) were administered during 11 days of multiple-dose treatment with 5 mg b.i.d. isradipine. Neither racemic warfarin nor isradipine binding to plasma proteins in vitro was altered by the addition of the other drug.
Digoxin: The concomitant administration of isradipine and digoxin in a single-dose pharmacokinetic study did not affect renal, nonrenal and total body clearance of digoxin.
Fentanyl Anesthesia: Severe hypotension has been reported during fentanyl anesthesia with concomitant use of a beta-blocker and a calcium channel blocker. Even though such interactions have not been seen in clinical studies with isradipine, an increased volume of circulating fluids might be required if such an interaction were to occur.
Treatment of male rats for 2 years with 2.5, 12.5, or 62.5 mg/kg/day isradipine admixed with the diet (approximately 6, 31, and 156 times the maximum recommended daily dose based on a 50 kg man) resulted in dose dependent increases in the incidence of benign Leydig cell tumors and testicular hyperplasia relative to untreated control animals. These findings, which were replicated in a subsequent experiment, may have been indirectly related to an effect of isradipine on circulating gonadotropin levels in the rats; a comparable endocrine effect was not evident in male patients receiving therapeutic doses of the drug on a chronic basis. Treatment of mice for two years with 2.5, 15, or 80 mg/kg/day isradipine in the diet (approximately 6, 38, and 200 times the maximum recommended dose based on a 50 kg man) showed no evidence of oncogenicity. There was no evidence of mutagenic potential based on the results of a battery of mutagenic tests. No effect on fertility was observed in male and female rats treated with up to 60 mg/kg/day isradipine.
Pregnancy Category C: Isradipine was administered orally to rats and rabbits during organogenesis. Treatment of pregnant rats with doses of 6, 20, or 60 mg/kg/day produced a significant reduction in maternal weight gain during treatment with the highest dose (150 times the maximum recommended human daily dose) but with no lasting effects on the mother or the offspring. Treatment of pregnant rabbits with doses of 1, 3, or 10 mg/kg/day (2.5, 7.5, and 25 times the maximum recommended human daily dose) produced decrements in maternal body weight gain and increased fetal resorption at the two higher doses. There was no evidence of embryotoxicity at doses which were not maternotoxic and no evidence of teratogenicity at any dose tested. In a peri/postnatal administration study in rats, reduced maternal body weight gain during late pregnancy at oral doses of 20 and 60 mg/kg/day isradipine was associated with reduced birth weights and decreased peri and postnatal pup survival.
There are no adequate and well controlled studies in pregnant women. The use of isradipine during pregnancy should only be considered if the potential benefit outweighs potential risks.
It is not known whether isradipine is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for adverse effects of isradipine on nursing infants, a decision should be made as to whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Safety and effectiveness in pediatric patients have not been established.
In multiple dose U.S. studies in hypertension, 1228 patients received isradipine alone or in combination with other agents, principally a thiazide diuretic, 934 of them in controlled comparisons with placebo or active agents. An additional 652 patients (which includes 374 normal volunteers) received isradipine in U.S. studies of conditions other than hypertension, and 1321 patients received isradipine in non-U.S. studies. About 500 patients received isradipine in long-term hypertension studies, 410 of them for at least 6 months. The adverse reaction rates given below are principally based on controlled hypertension studies, but rarer serious events are derived from all exposures to isradipine, including foreign marketing experience.
Most adverse reactions were mild and related to the vasodilatory effects of isradipine (dizziness, edema, palpitations, flushing, tachycardia), and many were transient. About 5% of isradipine patients left studies prematurely because of adverse reactions (vs. 3% of placebo patients and 6% of active control patients), principally due to headache, edema, dizziness, palpitations, and gastrointestinal disturbances.
The following table shows the most common adverse reactions, volunteered or elicited, considered by the investigator to be at least possibly drug related. The results for the isradipine treated patients are presented for all doses pooled together (reported by 1% or greater of patients receiving any dose of isradipine), and also for the two treatment regimens most applicable to the treatment of hypertension with isradipine: (1) initial and maintenance dose of 2.5 mg b.i.d., and (2) initial dose of 2.5 mg b.i.d. followed by maintenance dose of 5 mg b.i.d.
| Isradipine |
||||||
|
Adverse |
All Doses N=934 % |
2.5 mg b.i.d. 199 % |
5 mg b.i.d.† 150 % |
10 mg b.i.d.†† 59 % |
Placebo % |
Active Controls* 414 % |
| Headache Dizziness Edema Palpitations |
13.7 7.3 7.2 4.0 |
12.6 8.0 3.5 1.0 |
10.7 5.3 8.7 4.7 |
22.0 3.4 8.5 5.1 |
14.1 4.4 3.0 1.4 |
9.4 8.2 2.9 1.5 |
| Fatigue Flushing Chest Pain Nausea |
3.9 2.6 2.4 1.8 |
2.5 3.0 2.5 1.0 |
2.0 2.0 2.7 2.7 |
8.5 5.1 1.7 5.1 |
0.3 0.0 2.4 1.7 |
6.3 1.2 2.9 3.1 |
| Dyspnea Abdominal Discomfort Tachycardia Rash |
1.8 |
0.5 0.0 1.0 1.5 |
2.7 3.3 1.3 2.0 |
3.4 1.7 3.4 1.7 |
1.0 1.7 0.3 0.3 |
2.2 3.9 0.5 0.7 |
| Pollakiuria Weakness Vomiting Diarrhea |
1.5 1.2 1.1 1.1 |
2.0 0.0 1.0 0.0 |
1.3 0.7 1.3 2.7 |
3.4 0.0 0.0 3.4 |
0.0 0.0 0.3 2.0 |
<1.0 1.2 0.2 1.9 |
†Initial dose of 2.5 mg b.i.d. followed by maintenance dose of 5 mg b.i.d.
†† Initial dose of 2.5 mg b.i.d. followed by sequential titration to 5 mg b.i.d., 7.5 mg b.i.d., and maintenance dose of 10 mg b.i.d.
*Propranolol, prazosin, hydrochlorothiazide, enalapril, captopril.
Except for headache, which is not clearly drug-related (see previous table), the more frequent adverse reactions listed show little change, or increase slightly, in frequency over time, as shown in the following table:
| Incidence Rates for Isradipine (All Doses) by Week (%) |
||||||
|
Week N |
1 694 |
2 906 |
3 649 |
4 847 |
5 432 |
6 494 |
| Adverse Reaction | ||||||
| Headache Dizziness Edema Palpitations Fatigue Flushing |
6.5 1.6 1.2 1.2 0.4 1.2 |
6.1 1.9 2.5 1.3 1.0 1.3 |
5.2 1.7 3.2 1.4 1.4 2.0 |
5.2 2.2 3.2 1.9 1.2 1.4 |
5.8 2.3 5.3 2.1 1.2 2.1 |
4.5 2.0 5.5 1.4 1.6 1.4 |
|
Week N |
7 153 |
8 377 |
9 261 |
10 362 |
11 107 |
12 105 |
| Adverse Reaction | ||||||
| Headache Dizziness Edema Palpitations Fatigue Flushing |
2.0 2.0 5.9 1.3 2.0 3.3 |
2.7 1.9 5.0 0.8 2.7 1.3 |
1.9 2.3 4.6 0.8 1.5 1.1 |
2.8 3.9 4.7 1.7 1.4 0.8 |
2.8 4.7 3.8 1.9 0.9 0.0 |
3.8 3.8 3.8 2.9 1.9 0.0 |
Edema, palpitations, fatigue, and flushing appear to be dose-related, especially at the higher doses of 15-20 mg/day.
In open-label, long-term studies of up to two years in duration, the adverse events reported were generally the same as those reported in the short-term controlled trials. The overall frequencies of these adverse events were slightly higher in the long-term than in the controlled studies, but as in the controlled trials most adverse reactions were mild and transient.
The following adverse experiences were reported in 0.5%-1% of the isradipine-treated patients in hypertensive studies, or are rare. More serious events from this and other data sources, including postmarketing exposure, are shown in italics. The relationship of these adverse events to isradipine administration is uncertain.
Skin: pruritus, urticaria
Musculoskeletal: cramps of legs/feet
Respiratory: cough
Cardiovascular: shortness of breath, hypotension, atrial fibrillation, ventricular fibrillation, myocardial infarction, heart failure
Gastrointestinal: abdominal discomfort, constipation, diarrhea
Urogenital: nocturia
Nervous System: drowsiness, insomnia, lethargy, nervousness, impotence, decreased libido, depression, syncope, paresthesia (which includes numbness and tingling), transient ischemic attack, stroke
Autonomic: hyperhidrosis, visual disturbance, dry mouth, numbness
Miscellaneous: throat discomfort, leukopenia, elevated liver function tests.
Minimal empirical data are available on isradipine overdosage. Three individual suicide attempts with dosages of isradipine reported to be from 20 mg up to 100 mg resulted in lethargy, sinus tachycardia and, in the case of the person ingesting 100 mg, transient hypotension which responded to fluid therapy. A foreign report of the ingestion of 200 mg of isradipine with ethanol resulted only in flushing, tachycardia with ST depression on ECG, and hypotension, all of which were reversible. The ingestion of 5 mg isradipine by a 22-month child and the accidental ingestion of 100 mg of isradipine by a 58-year old female did not result in any sequelae.
Available data suggest that, as with other dihydropyridines, overdosage with isradipine might result in excessive peripheral vasodilation with subsequent marked and probably prolonged systemic hypotension, and tachycardia. Emesis, gastric lavage, administration of activated charcoal followed in 30 minutes by a saline cathartic would be reasonable therapy. Isradipine is highly protein-bound and not removed by hemodialysis. Overdosage characterized by clinically significant hypotension should be treated with active cardiovascular support including monitoring of cardiac and respiratory function, elevation of lower extremities, and attention to circulating fluid volume and urine output. A vasoconstrictor (such as epinephrine, norepinephrine, or levarterenol) may be helpful in restoring a normotensive state, provided that there is no contraindication to its use.
Refractory hypotension or AV conduction disturbances may be treated with intravenous calcium salts, or glucagon. Cimetidine should be withheld in such instances due to the risk of further increasing plasma isradipine levels.
Significant lethality was observed in mice given oral doses of over 200 mg/kg and rabbits given about 50 mg/kg of isradipine. Rats tolerated doses of over 2000 mg/kg without effects on survival.
The dosage of isradipine should be individualized. The recommended initial dose of isradipine is 2.5 mg b.i.d. alone or in combination with a thiazide diuretic. An antihypertensive response usually occurs within 2-3 hours. Maximal response may require 2-4 weeks. If a satisfactory reduction in blood pressure does not occur after this period, the dose may be adjusted in increments of 5 mg/day at 2-4 week intervals up to a maximum of 20 mg/day. Most patients, however, show no additional response to doses above 10 mg/day, and adverse effects are increased in frequency above 10 mg/day.
The bioavailability of isradipine (increased AUC) is increased in elderly patients (above 65 years of age), patients with hepatic functional impairment, and patients with mild renal impairment. Ordinarily, the starting dose should still be 2.5 mg b.i.d. in these patients.
Isradipine Capsules, USP
2.5 mg
Brown opaque, imprinted with “ ” IS 2.5.
” IS 2.5.
NDC 50268-454-15 10 tablets per card, 5 cards per carton.
5 mg
Caramel opaque, imprinted with “ ” IS 5.
” IS 5.
NDC 50268-455-15 10 tablets per card, 5 cards per carton.
Store and Dispense
Dispensed in blister punch material for Institutional Use Only.
Store at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature] and dispense in a tight, light resistant container.
Rx only
Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478
Mfg. Ref. 8010800/0508
AvPAK
AV 01/14 ( P)
NDC 50268-454-15
Isradipine Capsule, USP
2.5 mg
Rx Only
50 Capsules (5 X 10) Unit Dose
5026845415
NDC 50268-454-15
Isradipine Capsule, USP
2.5 mg
Rx Only
50 Capsules (5 X 10) Unit Dose
5026845415
See package insert for dosage information.
Store at 20o-25oC (68o-77oF) [See USP Controlled Room Temperature] and dispense in a tight, light-resistant container.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478
AvPAK
A PRODUCT OF AvKARE
Mfg. Iss. 10/08 AV 12/13 (P)
NDC 50268-455-15
Isradipine Capsule, USP
5 mg
Rx Only
50 Capsules (5 X 10) Unit Dose
5026845515
NDC 50268-455-15
Isradipine Capsule, USP
5 mg
Rx Only
50 Capsules (5 X 10) Unit Dose
5026845515
See package insert for dosage information.
Store at 20o-25oC (68o-77oF) [See USP Controlled Room Temperature] and dispense in a tight, light-resistant container.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
Manufactured for:
AvKARE, Inc.
Pulaski, TN 38478
AvPAK
A PRODUCT OF AvKARE
Mfg. Iss. 10/08 AV 12/13 (P)
IsradipineIsradipine CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
IsradipineIsradipine CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||