ISTODAX
Celgene Corporation
Celgene Corporation
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ISTODAX safely and effectively. See full prescribing information for ISTODAX. ISTODAX (romidepsin) for injection For intravenous infusion only Initial U.S. Approval: 2009INDICATIONS AND USAGEISTODAX is a histone deacetylase (HDAC) inhibitor indicated for: Treatment of cutaneous T-cell lymphoma (CTCL) in patients who have received at least one prior systemic therapy (1). Treatment of peripheral T-cell lymphoma (PTCL) in patients who have received at least one prior therapy (1). These indications are based on response rate. Clinical benefit such as improvement in overall survival has not been demonstrated (1).DOSAGE AND ADMINISTRATION 14 mg/m2 administered intravenously (IV) over a 4-hour period on days 1, 8, and 15 of a 28-day cycle. Repeat cycles every 28 days provided that the patient continues to benefit from and tolerates the drug (2.1). Treatment discontinuation or interruption with or without dose reduction to 10 mg/m2 may be needed to manage adverse drug reactions (2.2). DOSAGE FORMS AND STRENGTHSISTODAX for injection, 10 mg, supplied with one Diluent vial containing 2 mL (deliverable volume) of solution (3).CONTRAINDICATIONSNone.WARNINGS AND PRECAUTIONS Treatment with ISTODAX has been associated with thrombocytopenia, leukopenia (neutropenia and lymphopenia), and anemia; therefore, monitor these hematological parameters during treatment with ISTODAX, modify the dose as necessary (5.1). Serious and sometimes fatal infections have been reported during treatment and within 30 days after treatment with ISTODAX (5.2). Electrocardiographic (ECG) changes have been observed. Consider cardiovascular monitoring precautions in patients with congenital long QT syndrome, a history of significant cardiovascular disease, and patients taking medicinal products that lead to significant QT prolongation. Ensure that potassium and magnesium are within the normal range before administration of ISTODAX (5.3). Tumor lysis syndrome has been reported during treatment with ISTODAX. Patients with advanced stage disease and/or high tumor burden should be closely monitored and appropriate precautions taken (5.4). ISTODAX may cause fetal harm when administered to a pregnant woman. Advise women of potential hazard to the fetus and to avoid pregnancy while receiving Istodax (5.5, 8.1). Side EffectsThe most common adverse reactions were neutropenia, lymphopenia, thrombocytopenia, infections, nausea, fatigue, vomiting, anorexia, anemia, and ECG T-wave changes (6). To report SUSPECTED ADVERSE REACTIONS, contact Celgene Corporation at 1-888-423-5436 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Carefully monitor prothrombin time (PT) and International Normalized Ratio (INR) in patients concurrently administered ISTODAX and Coumadin derivatives (7.1). Monitor for toxicities related to increased romidepsin exposure when co-administering romidepsin with strong CYP3A4 inhibitors (7.2). Avoid use with rifampin and strong CYP3A4 inducers (7.3).
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 ISTODAX INDICATIONS AND USAGE
- 2 ISTODAX DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 ISTODAX CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 ISTODAX ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 ISTODAX DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
ISTODAX is indicated for:
- Treatment of cutaneous T-cell lymphoma (CTCL) in patients who have received at least one prior systemic therapy.
- Treatment of peripheral T-cell lymphoma (PTCL) in patients who have received at least one prior therapy.
These indications are based on response rate. Clinical benefit such as improvement in overall survival has not been demonstrated.
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
The recommended dose of romidepsin is 14 mg/m2 administered intravenously over a 4-hour period on days 1, 8, and 15 of a 28-day cycle. Cycles should be repeated every 28 days provided that the patient continues to benefit from and tolerates the drug.
2.2 Dose Modification
Nonhematologic toxicities except alopecia
- Grade 2 or 3 toxicity: Treatment with romidepsin should be delayed until toxicity returns to ≤ Grade 1 or baseline, then therapy may be restarted at 14 mg/m2. If Grade 3 toxicity recurs, treatment with romidepsin should be delayed until toxicity returns to ≤ Grade 1 or baseline and the dose should be permanently reduced to 10 mg/m2.
- Grade 4 toxicity: Treatment with romidepsin should be delayed until toxicity returns to ≤ Grade 1 or baseline, then the dose should be permanently reduced to 10 mg/m2.
- Romidepsin should be discontinued if Grade 3 or 4 toxicities recur after dose reduction.
Hematologic toxicities
- Grade 3 or 4 neutropenia or thrombocytopenia: Treatment with romidepsin should be delayed until the specific cytopenia returns to ANC ≥1.5×109/L and/or platelet count ≥75×109/L or baseline, then therapy may be restarted at 14 mg/m2.
- Grade 4 febrile (≥38.5ºC) neutropenia or thrombocytopenia that requires platelet transfusion: Treatment with romidepsin should be delayed until the specific cytopenia returns to ≤ Grade 1 or baseline, and then the dose should be permanently reduced to 10 mg/m2.
2.3 Instructions for Preparation and Intravenous Administration
ISTODAX should be handled in a manner consistent with recommended safe procedures for handling cytotoxic drugs.
ISTODAX must be reconstituted with the supplied diluent and further diluted with 0.9% Sodium Chloride Injection, USP before intravenous infusion.
- Each 10 mg single-use vial of ISTODAX (romidepsin) must be reconstituted with 2 mL of the supplied Diluent. With a suitable syringe, aseptically withdraw 2 mL from the supplied Diluent vial, and slowly inject it into the ISTODAX (romidepsin) for injection vial. Swirl the contents of the vial until there are no visible particles in the resulting solution. The reconstituted solution will contain ISTODAX 5 mg/mL. The reconstituted ISTODAX solution is chemically stable for at least 8 hours at room temperature.
- Extract the appropriate amount of ISTODAX from the vials to deliver the desired dose, using proper aseptic technique. Before intravenous infusion, further dilute ISTODAX in 500 mL 0.9% Sodium Chloride Injection, USP.
- Infuse over 4 hours.
The diluted solution is compatible with polyvinyl chloride (PVC), ethylene vinyl acetate (EVA), polyethylene (PE) infusion bags as well as glass bottles, and is chemically stable for at least 24 hours when stored at room temperature. However, it should be administered as soon after dilution as possible.
Parenteral drug products should be inspected visually for particulate matter and discoloration before administration, whenever solution and container permit.
3 DOSAGE FORMS AND STRENGTHS
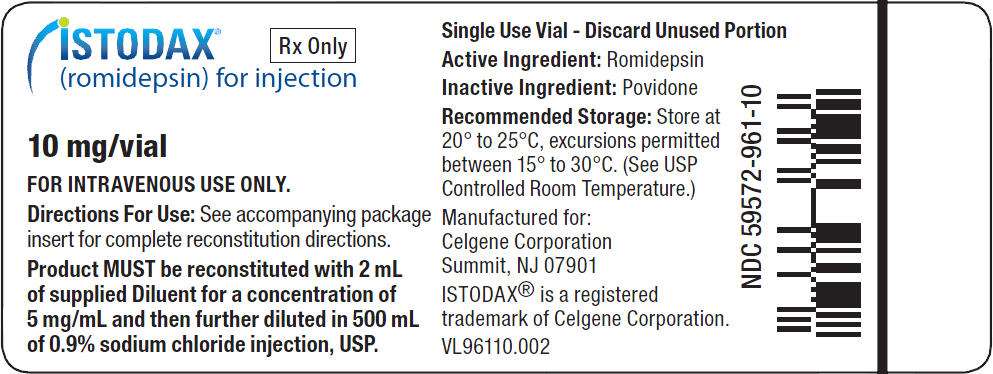
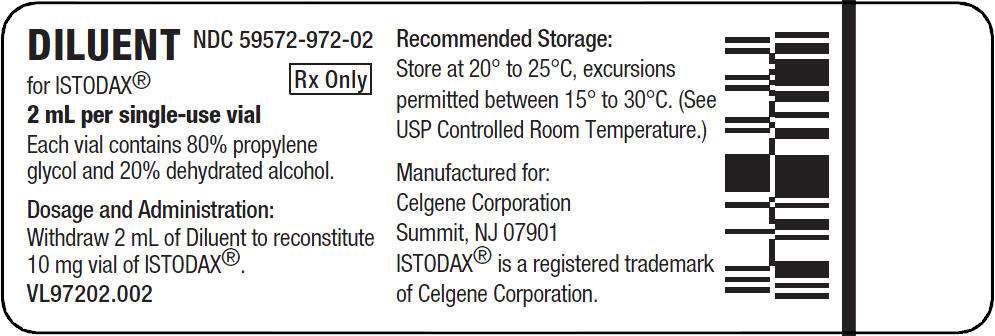
ISTODAX is supplied as a kit which includes a sterile, lyophilized powder in a single-use vial containing 10 mg of romidepsin and 20 mg of the bulking agent, povidone, USP. In addition, each kit includes 1 sterile vial containing 2 mL (deliverable volume) of the Diluent composed of 80% propylene glycol, USP, and 20% dehydrated alcohol, USP.
4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Hematologic
Treatment with ISTODAX can cause thrombocytopenia, leukopenia (neutropenia and lymphopenia), and anemia; therefore, these hematological parameters should be monitored during treatment with ISTODAX, and the dose should be modified, as necessary [See Dosage and Administration (2.2) and Adverse Reactions (6)].
5.2 Infection
Serious and sometimes fatal infections, including pneumonia and sepsis, have been reported in clinical trials with ISTODAX. These can occur during treatment and within 30 days after treatment, and the risk of life threatening infections may be higher in patients with a history of extensive or intensive chemotherapy [See Adverse Reactions (6)].
5.3 Electrocardiographic Changes
Several treatment-emergent morphological changes in ECGs (including T-wave and ST-segment changes) have been reported in clinical studies. The clinical significance of these changes is unknown [See Adverse Reactions (6)].
In patients with congenital long QT syndrome, patients with a history of significant cardiovascular disease, and patients taking anti-arrhythmic medicines or medicinal products that lead to significant QT prolongation, appropriate cardiovascular monitoring precautions should be considered, such as the monitoring of electrolytes and ECGs at baseline and periodically during treatment.
Potassium and magnesium should be within the normal range before administration of ISTODAX [See Adverse Reactions (6)].
5.4 Tumor Lysis Syndrome
Tumor lysis syndrome (TLS) has been reported to occur in 1% of patients with tumor stage CTCL and 2% of patients with Stage III/IV PTCL. Patients with advanced stage disease and/or high tumor burden should be closely monitored, appropriate precautions should be taken, and treatment should be instituted as appropriate.
5.5 Use in Pregnancy
There are no adequate and well-controlled studies of ISTODAX in pregnant women. However, based on its mechanism of action and findings in animals, ISTODAX may cause fetal harm when administered to a pregnant woman. In an animal reproductive study, romidepsin was embryocidal and resulted in adverse effects on the developing fetus at exposures below those in patients at the recommended dose of 14 mg/m2/week. If this drug is used during pregnancy, or if the patient becomes pregnant while taking ISTODAX, the patient should be apprised of the potential hazard to the fetus [See Use in Specific Populations (8.1)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Cutaneous T-Cell Lymphoma
The safety of ISTODAX was evaluated in 185 patients with CTCL in 2 single arm clinical studies in which patients received a starting dose of 14 mg/m2. The mean duration of treatment in these studies was 5.6 months (range: <1 to 83.4 months).
Common Adverse Reactions
Table 1 summarizes the most frequent adverse reactions (> 20%) regardless of causality using the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE, Version 3.0). Due to methodological differences between the studies, the AE data are presented separately for Study 1 and Study 2. Adverse reactions are ranked by their incidence in Study 1. Laboratory abnormalities commonly reported (>20%) as adverse reactions are included in Table 1.
| Adverse Reactions n (%) |
Study 1 (n=102) |
Study 2 (n=83) |
||
| All | Grade 3 or 4 | All | Grade 3 or 4 | |
| Any adverse reaction | 99 (97) | 36 (35) | 83 (100) | 68 (82) |
| Nausea | 57 (56) | 3 (3) | 71 (86) | 5 (6) |
| Asthenia/Fatigue | 54 (53) | 8 (8) | 64 (77) | 12 (14) |
| Infections | 47 (46) | 11 (11) | 45 (54) | 27 (33) |
| Vomiting | 35 (34) | 1 (<1) | 43 (52) | 8 (10) |
| Anorexia | 23 (23) | 1 (<1) | 45 (54) | 3 (4) |
| Hypomagnesemia | 22 (22) | 1 (<1) | 23 (28) | 0 |
| Diarrhea | 20 (20) | 1 (<1) | 22 (27) | 1 (1) |
| Pyrexia | 20 (20) | 4 (4) | 19 (23) | 1 (1) |
| Anemia | 19 (19) | 3 (3) | 60 (72) | 13 (16) |
| Thrombocytopenia | 17 (17) | 0 | 54 (65) | 12 (14) |
| Dysgeusia | 15 (15) | 0 | 33 (40) | 0 |
| Constipation | 12 (12) | 2 (2) | 32 (39) | 1 (1) |
| Neutropenia | 11 (11) | 4 (4) | 47 (57) | 22 (27) |
| Hypotension | 7 (7) | 3 (3) | 19 (23) | 3 (4) |
| Pruritus | 7 (7) | 0 | 26 (31) | 5 (6) |
| Hypokalemia | 6 (6) | 0 | 17 (20) | 2 (2) |
| Dermatitis/Exfoliative dermatitis | 4 (4) | 1 (<1) | 22 (27) | 7 (8) |
| Hypocalcemia | 4 (4) | 0 | 43 (52) | 5 (6) |
| Leukopenia | 4 (4) | 0 | 38 (46) | 18 (22) |
| Lymphopenia | 4 (4) | 0 | 47 (57) | 31 (37) |
| Alanine aminotransferase increased | 3 (3) | 0 | 18 (22) | 2 (2) |
| Aspartate aminotransferase increased | 3 (3) | 0 | 23 (28) | 3 (4) |
| Hypoalbuminemia | 3 (3) | 1 (<1) | 40 (48) | 3 (4) |
| Electrocardiogram ST-T wave changes | 2 (2) | 0 | 52 (63) | 0 |
| Hyperglycemia | 2 (2) | 2 (2) | 42 (51) | 1 (1) |
| Hyponatremia | 1 (<1) | 1 (<1) | 17 (20) | 2 (2) |
| Hypermagnesemia | 0 | 0 | 22 (27) | 7 (8) |
| Hypophosphatemia | 0 | 0 | 22 (27) | 8 (10) |
| Hyperuricemia | 0 | 0 | 27 (33) | 7 (8) |
Serious Adverse Reactions
Infections were the most common type of SAE reported in both studies with 8 patients (8%) in Study 1 and 26 patients (31%) in Study 2 experiencing a serious infection. Serious adverse reactions reported in > 2% of patients in Study 1 were sepsis and pyrexia (3%). In Study 2, serious adverse reactions in > 2% of patients were fatigue (7%), supraventricular arrhythmia, central line infection, neutropenia (6%), hypotension, hyperuricemia, edema (5%), ventricular arrhythmia, thrombocytopenia, nausea, leukopenia, dehydration, pyrexia, aspartate aminotransferase increased, sepsis, catheter related infection, hypophosphatemia and dyspnea (4%).
Most deaths were due to disease progression. In Study 1, there were two deaths due to cardiopulmonary failure and acute renal failure. In Study 2, there were six deaths due to infection (4), myocardial ischemia, and acute respiratory distress syndrome.
Discontinuations
Discontinuation due to an adverse event occurred in 21% of patients in Study 1 and 11% in Study 2. Discontinuations occurring in at least 2% of patients in either study included infection, fatigue, dyspnea, QT prolongation, and hypomagnesemia.
Peripheral T-Cell Lymphoma
The safety of ISTODAX was evaluated in 178 patients with PTCL in a sponsor-conducted pivotal study (Study 3) and a secondary NCI-sponsored study (Study 4) in which patients received a starting dose of 14 mg/m2. The mean duration of treatment and number of cycles in these studies were 5.6 months and 6 cycles.
Common Adverse Reactions
Table 2 summarizes the most frequent adverse reactions (≥ 10%) regardless of causality, using the NCI-CTCAE, Version 3.0. The AE data are presented separately for Study 3 and Study 4. Laboratory abnormalities commonly reported (≥ 10%) as adverse reactions are included in Table 2.
| Adverse Reactions n (%) |
Study 3 (N=131) |
Study 4 (N=47) |
||
| All | Grade 3 or 4 | All | Grade 3 or 4 | |
| Any adverse reactions | 127 (97) | 86 (66) | 47 (100) | 40 (85) |
| Gastrointestinal disorders | ||||
| Nausea | 77 (59) | 3 (2) | 35 (75) | 3 (6) |
| Vomiting | 51 (39) | 6 (5) | 19 (40) | 4 (9) |
| Diarrhea | 47 (36) | 3 (2) | 17 (36) | 1 (2) |
| Constipation | 39 (30) | 1 (<1) | 19 (40) | 1 (2) |
| Abdominal pain | 18 (14) | 3 (2) | 6 (13) | 1 (2) |
| Stomatitis | 13 (10) | 0 | 3 (6) | 0 |
| General disorders and administration site conditions | ||||
| Asthenia/Fatigue | 72 (55) | 11 (8) | 36 (77) | 9 (19) |
| Pyrexia | 46 (35) | 7 (5) | 22 (47) | 8 (17) |
| Chills | 14 (11) | 1 (<1) | 8 (17) | 0 |
| Edema peripheral | 13 (10) | 1 (<1) | 3 (6) | 0 |
| Blood and lymphatic system disorders | ||||
| Thrombocytopenia | 53 (41) | 32 (24) | 34 (72) | 17 (36) |
| Neutropenia | 39 (30) | 26 (20) | 31 (66) | 22 (47) |
| Anemia | 32 (24) | 14 (11) | 29 (62) | 13 (28) |
| Leukopenia | 16 (12) | 8 (6) | 26 (55) | 21 (45) |
| Metabolism and nutrition disorders | ||||
| Anorexia | 37 (28) | 2 (2) | 21 (45) | 1 (2) |
| Hypokalemia | 14 (11) | 3 (2) | 8 (17) | 1 (2) |
| Nervous system disorders | ||||
| Dysgeusia | 27 (21) | 0 | 13 (28) | 0 |
| Headache | 19 (15) | 0 | 16 (34) | 1 (2) |
| Respiratory, thoracic and mediastinal disorders | ||||
| Cough | 23 (18) | 0 | 10 (21) | 0 |
| Dyspnea | 17 (13) | 3 (2) | 10 (21) | 2 (4) |
| Investigations | ||||
| Weight decreased | 13 (10) | 0 | 7 (15) | 0 |
| Cardiac disorders | ||||
| Tachycardia | 13 (10) | 0 | 0 | 0 |
Serious Adverse Reactions
Infections were the most common type of SAE reported. In Study 3, 25 patients (19%) experienced a serious infection, including 6 patients (5%) with serious treatment-related infections. In Study 4, 11 patients (23%) experienced a serious infection, including 8 patients (17%) with serious treatment-related infections. Serious adverse reactions reported in ≥ 2% of patients in Study 3 were pyrexia (7%), pneumonia, sepsis, vomiting (5%), cellulitis, deep vein thrombosis, (4%), febrile neutropenia, abdominal pain (3%), chest pain, neutropenia, pulmonary embolism, dyspnea, and dehydration (2%). In Study 4, serious adverse reactions in ≥ 2 patients were pyrexia (17%), aspartate aminotransferase increased, hypotension (13%), anemia, thrombocytopenia, alanine aminotransferase increased (11%), infection, dehydration, dyspnea (9%), lymphopenia, neutropenia, hyperbilirubinemia, hypocalcemia, hypoxia (6%), febrile neutropenia, leukopenia, ventricular arrhythmia, vomiting, hypersensitivity, catheter related infection, hyperuricemia, hypoalbuminemia, syncope, pneumonitis, packed red blood cell transfusion, and platelet transfusion (4%).
Deaths due to all causes within 30 days of the last dose of ISTODAX occurred in 7% of patients in Study 3 and 17% of patients in Study 4. In Study 3, there were 5 deaths unrelated to disease progression that were due to infections, including multi-organ failure/sepsis, pneumonia, septic shock, candida sepsis, and sepsis/cardiogenic shock. In Study 4, there were 3 deaths unrelated to disease progression that were due to sepsis, aspartate aminotransferase elevation in the setting of Epstein Barr virus reactivation, and death of unknown cause.
Discontinuations
Discontinuation due to an adverse event occurred in 19% of patients in Study 3 and in 28% of patients in Study 4. In Study 3, thrombocytopenia and pneumonia were the only events leading to treatment discontinuation in at least 2% of patients. In Study 4, events leading to treatment discontinuation in ≥ 2 patients were thrombocytopenia (11%), anemia, infection, and alanine aminotransferase increased (4%).
6.2 Postmarketing Experience
No additional safety signals have been observed from postmarketing experience.
7 DRUG INTERACTIONS
7.1 Coumadin or Coumadin Derivatives
Prolongation of PT and elevation of INR were observed in a patient receiving ISTODAX concomitantly with warfarin. Although the interaction potential between ISTODAX and Coumadin or Coumadin derivatives has not been formally studied, physicians should carefully monitor PT and INR in patients concurrently administered ISTODAX and Coumadin or Coumadin derivatives [See Clinical Pharmacology (12.3)].
7.2 Drugs that Inhibit Cytochrome P450 3A4 Enzymes
Romidepsin is metabolized by CYP3A4. Strong CYP3A4 inhibitors increase concentrations of romidepsin. In a pharmacokinetic drug interaction trial the strong CYP3A4 inhibitor ketoconazole increased romidepsin (AUC0-∞) by approximately 25% [See Clinical Pharmacology (12.3)].
Monitor for toxicity related to increased romidepsin exposure and follow the dose modifications for toxicity [see Dosage and Administration (2.2) ] when romidepsin is initially co-administered with strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole).
7.3 Drugs that Induce Cytochrome P450 3A4 Enzymes
Avoid co-administration of ISTODAX with rifampin.
In a pharmacokinetic drug interaction trial with co-administered rifampin (a strong CYP3A4 inducer), romidepsin exposure was increased by approximately 80% and 60% for AUC0-∞ and Cmax, respectively [See Clinical Pharmacology (12.3)]. Typically, co-administration of CYP3A4 inducers decrease concentrations of drugs metabolized by CYP3A4. The increase in exposure seen after co-administration with rifampin is likely due to rifampin’s inhibition of an undetermined hepatic uptake process that is predominantly responsible for the disposition of ISTODAX.
It is unknown if other potent CYP3A4 inducers (e.g., dexamethasone, carbamazepine, phenytoin, rifabutin, rifapentine, phenobarbital, St. John’s Wort) would alter the exposure of ISTODAX. Therefore, the use of other potent CYP3A4 inducers should be avoided when possible.
7.4 Drugs that Inhibit Drug Transport Systems
Romidepsin is a substrate of the efflux transporter P-glycoprotein (P-gp, ABCB1). If ISTODAX is administered with drugs that inhibit P-gp, increased concentrations of romidepsin are likely, and caution should be exercised.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D [See Warnings and Precautions (5.5) ].
There are no adequate and well-controlled studies of ISTODAX in pregnant women. However, based on its mechanism of action and findings in animals, ISTODAX may cause fetal harm when administered to a pregnant woman. In an animal reproductive study, romidepsin was embryocidal and resulted in adverse effects on the developing fetus at exposures below those in patients at the recommended dose. If this drug is used during pregnancy, or if the patient becomes pregnant while taking ISTODAX, the patient should be apprised of the potential hazard to the fetus.
Romidepsin was administered intravenously to rats during the period of organogenesis at doses of 0.1, 0.2, or 0.5 mg/kg/day. Substantial resorption or post-implantation loss was observed at the high-dose of 0.5 mg/kg/day, a maternally toxic dose. Adverse embryo-fetal effects were noted at romidepsin doses of ≥0.1 mg/kg/day, with systemic exposures (AUC) ≥0.2% of the human exposure at the recommended dose of 14 mg/m2/week. Drug-related fetal effects consisted of folded retina, rotated limbs, and incomplete sternal ossification.
8.3 Nursing Mothers
It is not known whether romidepsin is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ISTODAX, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of ISTODAX in pediatric patients has not been established.
8.5 Geriatric Use
Of the approximately 300 patients with CTCL or PTCL in trials, about 25% were >65 years old. No overall differences in safety or effectiveness were observed between these subjects and younger subjects; however, greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
No dedicated hepatic impairment study for ISTODAX has been conducted. Mild hepatic impairment does not alter pharmacokinetics of romidepsin based on a population pharmacokinetic analysis. Patients with moderate and severe hepatic impairment should be treated with caution [See Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No dedicated renal impairment study for ISTODAX has been conducted. Based upon the population pharmacokinetic analysis, renal impairment is not expected to significantly influence drug exposure. The effect of end-stage renal disease on romidepsin pharmacokinetics has not been studied. Thus, patients with end-stage renal disease should be treated with caution [See Clinical Pharmacology (12.3)].
10 OVERDOSAGE
No specific information is available on the treatment of overdosage of ISTODAX.
Toxicities in a single-dose study in rats or dogs, at intravenous romidepsin doses up to 2.2 fold the recommended human dose based on the body surface area, included irregular respiration, irregular heart beat, staggering gait, tremor, and tonic convulsions.
In the event of an overdose, it is reasonable to employ the usual supportive measures, e.g., clinical monitoring and supportive therapy, if required. There is no known antidote for ISTODAX and it is not known if ISTODAX is dialyzable.
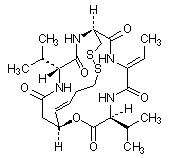
11 DESCRIPTION
Romidepsin, a histone deacetylase (HDAC) inhibitor, is a bicyclic depsipeptide. At room temperature, romidepsin is a white powder and is described chemically as (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(1-methylethyl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone. The empirical formula is C24H36N4O6S2.
The molecular weight is 540.71 and the structural formula is:
ISTODAX (romidepsin) for injection is intended for intravenous infusion only after reconstitution with the supplied Diluent and after further dilution with 0.9% Sodium Chloride, USP.
ISTODAX is supplied as a kit containing two vials.
ISTODAX (romidepsin) for injection is a sterile lyophilized white powder and is supplied in a single-use vial containing 10 mg romidepsin and 20 mg povidone, USP.
Diluent for ISTODAX is a sterile clear solution and is supplied in a single-use vial containing a 2-mL deliverable volume. Diluent for ISTODAX contains 80% (v/v) propylene glycol, USP and 20% (v/v) dehydrated alcohol, USP.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Romidepsin is a histone deacetylase (HDAC) inhibitor. HDACs catalyze the removal of acetyl groups from acetylated lysine residues in histones, resulting in the modulation of gene expression. HDACs also deacetylate non-histone proteins, such as transcription factors. In vitro, romidepsin causes the accumulation of acetylated histones, and induces cell cycle arrest and apoptosis of some cancer cell lines with IC50 values in the nanomolar range. The mechanism of the antineoplastic effect of romidepsin observed in nonclinical and clinical studies has not been fully characterized.
12.2 Pharmacodynamics
Cardiac Electrophysiology
The effect of romidepsin on the heart-rate corrected QTc/QTcF was evaluated in 26 subjects with advanced malignancies given romidepsin at doses of 14 mg/m2 as a 4-hour intravenous infusion, and at doses of 8, 10 or 12 mg/m2 as a 1–hour infusion. Patients received premedications with antiemetics. No large changes in the mean QTc interval (> 20 milliseconds) from baseline based on Fridericia correction method were detected in the trial. Small increase in mean QT interval (< 10 milliseconds) and mean QT interval increase between 10 to 20 milliseconds cannot be excluded because of the limitations in the trial design.
Romidepsin was associated with a delayed concentration-dependent increase in heart rate in patients with advanced cancer with a maximum mean increase in heart rate of 20 beats per minute occurring at the 6 hour time point after start of romidepsin infusion for patients receiving 14 mg/m2 as a 4-hour infusion.
12.3 Pharmacokinetics
Absorption
Romidepsin exhibited linear pharmacokinetics across doses ranging from 1.0 to 24.9 mg/m2 when administered intravenously over 4 hours in patients with advanced cancers.
In patients with T-cell lymphomas who received 14 mg/m2 of romidepsin intravenously over a 4-hour period on days 1, 8, and 15 of a 28-day cycle, geometric mean values of the maximum plasma concentration (Cmax) and the area under the plasma concentration versus time curve (AUC0-inf) were 377 ng/mL and 1549 ng*hr/mL, respectively.
Distribution
Romidepsin is highly protein bound in plasma (92% to 94%) over the concentration range of 50 ng/mL to 1000 ng/mL with α1-acid-glycoprotein (AAG) being the principal binding protein. Romidepsin is a substrate of the efflux transporter P-glycoprotein (P-gp, ABCB1).
In vitro, romidepsin accumulates into human hepatocytes via an unknown active uptake process. Romidepsin is not a substrate of the following uptake transporters: BCRP, BSEP, MRP2, OAT1, OAT3, OATP1B1, OATP1B3, or OCT2. In addition, romidepsin is not an inhibitor of BCRP, MRP2, MDR1 or OAT3. Although romidepsin did not inhibit OAT1, OCT2, and OATP1B3 at concentrations seen clinically (1 μmol/L), modest inhibition was observed at 10 umol/L. Romidepsin was found to be an inhibitor of BSEP and OATP1B1.
Metabolism
Romidepsin undergoes extensive metabolism in vitro primarily by CYP3A4 with minor contribution from CYP3A5, CYP1A1, CYP2B6, and CYP2C19. At therapeutic concentrations, romidepsin did not competitively inhibit CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4 in vitro.
At therapeutic concentrations, romidepsin did not cause notable induction of CYP1A2, CYP2B6 and CYP3A4 in vitro. Therefore, pharmacokinetic drug-drug interactions are unlikely to occur due to CYP450 induction or inhibition by romidepsin when co-administered with CYP450 substrates.
Excretion
Following 4-hour intravenous administration of romidepsin at 14 mg/m2 on days 1, 8, and 15 of a 28-day cycle in patients with T-cell lymphomas, the terminal half-life (t1/2) was approximately 3 hours. No accumulation of plasma concentration of romidepsin was observed after repeated dosing.
Drug Interactions
Ketoconazole: A drug interaction clinical trial with the strong CYP3A4 inhibitor, ketoconazole, was conducted in patients with advanced cancer. Following co-administration of 8 mg/m2 ISTODAX (4-hour infusion) with ketoconazole, the overall romidepsin exposure was increased by approximately 25% and 10% for AUC0-∞ and Cmax, respectively, compared to romidepsin alone, and the difference between the two treatments was statistically significant. Co-administration of ketoconazole slightly decreased the romidepsin clearance and volume of distribution, but did not have a statistically significant effect on peak exposure (Cmax). [See Drug Interactions (7.2)]
Rifampin: A drug interaction clinical trial with the strong CYP3A4 inducer, rifampin, was conducted in patients with advanced cancer. Following co-administration of 14 mg/m2 ISTODAX (4-hour infusion) with rifampin, the overall romidepsin exposure was unexpectedly increased by approximately 80% and 60% for AUC0-∞ and Cmax, respectively, compared to romidepsin alone, and the difference between the two treatments was statistically significant. Co-administration of rifampin decreased the romidepsin clearance and volume of distribution by 44% and 52%, respectively. The increase in exposure seen after co-administration with rifampin is likely due to rifampin's inhibition of a undetermined hepatic uptake process that is predominant for the disposition of ISTODAX. [See Drug Interactions (7.3)]
Use in Specific Populations
Effect of Age, Gender or Race
The population pharmacokinetic analysis of romidepsin showed that age, gender, or race (white vs. black) did not appear to influence the pharmacokinetics of romidepsin.
Effect of Hepatic Impairment
No dedicated hepatic impairment study has been conducted for ISTODAX. The population pharmacokinetic analysis indicates that mild hepatic impairment [total bilirubin (TB) ≤ upper limit of normal (ULN) and aspartate aminotransferase (AST) > ULN; or TB > 1.0x - 1.5x ULN and any AST] had no significant influence on romidepsin pharmacokinetics. As the effect of moderate (TB > 1.5x - 3x ULN and any AST) and severe (TB > 3x ULN and any AST) hepatic impairment on the pharmacokinetics of romidepsin is unknown, patients with moderate and severe hepatic impairment should be treated with caution [See Use in Specific Populations (8.6)].
Effect of Renal Impairment
No dedicated renal impairment study has been conducted for ISTODAX. The population pharmacokinetic analysis showed that romidepsin pharmacokinetics were not affected by mild (estimated creatinine clearance 50 - 80 mL/min), moderate (estimated creatinine clearance 30 - 50 mL/min), or severe (estimated creatinine clearance < 30 mL/min) renal impairment. Nonetheless, the effect of end-stage renal disease on romidepsin pharmacokinetics has not been studied. Thus, patients with end-stage renal disease should be treated with caution [See Use in Specific Populations (8.7)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been performed with romidepsin. Romidepsin was not mutagenic in vitro in the bacterial reverse mutation assay (Ames test) or the mouse lymphoma assay. Romidepsin was not clastogenic in an in vivo rat bone marrow micronucleus assay when tested to the maximum tolerated dose (MTD) of 1 mg/kg in males and 3 mg/kg in females (6 and 18 mg/m2 in males and females, respectively). These doses were up to 1.3-fold the recommended human dose, based on body surface area.
Based on non-clinical findings, male and female fertility may be compromised by treatment with ISTODAX. In a 26-week toxicology study, romidepsin administration resulted in testicular degeneration in rats at 0.33 mg/kg/dose (2 mg/m2/dose) following the clinical dosing schedule. This dose resulted in AUC0-inf. values that were approximately 2% the exposure level in patients receiving the recommended dose of 14 mg/m2/dose. A similar effect was seen in mice after 4 weeks of drug administration at higher doses. Seminal vesicle and prostate organ weights were decreased in a separate study in rats after 4 weeks of daily drug administration at 0.1 mg/kg/day (0.6 mg/m2/day), approximately 30% the estimated human daily dose based on body surface area. Romidepsin showed high affinity for binding to estrogen receptors in pharmacology studies. In a 26-week toxicology study in rats, atrophy was seen in the ovary, uterus, vagina and mammary gland of females administered doses as low as 0.1 mg/kg/dose (0.6 mg/m2/dose) following the clinical dosing schedule. This dose resulted in AUC0-inf. values that were 0.3% of those in patients receiving the recommended dose of 14 mg/m2/dose. Maturation arrest of ovarian follicles and decreased weight of ovaries were observed in a separate study in rats after four weeks of daily drug administration at 0.1 mg/kg/day (0.6 mg/m2/day). This dose is approximately 30% the estimated human daily dose based on body surface area.
14 CLINICAL STUDIES
14.1 Cutaneous T-Cell Lymphoma
ISTODAX was evaluated in 2 multicenter, single-arm clinical studies in patients with CTCL. Overall, 167 patients with CTCL were treated in the US, Europe, and Australia. Study 1 included 96 patients with confirmed CTCL after failure of at least 1 prior systemic therapy. Study 2 included 71 patients with a primary diagnosis of CTCL who received at least 2 prior skin directed therapies or one or more systemic therapies. Patients were treated with ISTODAX at a starting dose of 14 mg/m2 infused over 4 hours on days 1, 8, and 15 every 28 days.
In both studies, patients could be treated until disease progression at the discretion of the investigator and local regulators. Objective disease response was evaluated according to a composite endpoint that included assessments of skin involvement, lymph node and visceral involvement, and abnormal circulating T-cells ("Sézary cells").
The primary efficacy endpoint for both studies was overall objective disease response rate (ORR) based on the investigator assessments, and defined as the proportion of patients with confirmed complete response (CR) or partial response (PR). CR was defined as no evidence of disease and PR as ≥50% improvement in disease. Secondary endpoints in both studies included duration of response and time to response.
Baseline Patient Characteristics
Demographic and disease characteristics of the patients in Study 1 and Study 2 are provided in Table 3.
| Characteristic | Study 1 (N=96) |
Study 2 (N=71) |
|---|---|---|
| Age | ||
| N | 96 | 71 |
| Mean (SD) | 57 (12) | 56 (13) |
| Median (Range) | 57 (21, 89) | 57 (28, 84) |
| Sex, n (%) | ||
| Men | 59 (61) | 48 (68) |
| Women | 37 (39) | 23 (32) |
| Race, n (%) | ||
| White | 90 (94) | 55 (77) |
| Black | 5 ( 5) | 15 (21) |
| Other/Not Reported | 1 ( 1) | 1 ( 1) |
| Stage of Disease at Study Entry, n (%) | ||
| IA | 0 ( 0) | 1 ( 1) |
| IB | 15 (16) | 6 ( 9) |
| IIA | 13 (14) | 2 ( 3) |
| IIB | 21 (22) | 14 (20) |
| III | 23 (24) | 9 (13) |
| IVA | 24 (25) | 27 (38) |
| IVB | 0 ( 0) | 12 (17) |
| Number of Prior Skin-Directed Therapies | ||
| Median (Range) | 2 (0,6) | 1 (0,3) |
| Number of Prior Systemic Therapies | ||
| Median (Range) | 2 (1, 8) | 2 (0, 7) |
Clinical Results
Efficacy outcomes for CTCL patients are provided in Table 4. Median time to first response was 2 months (range 1 to 6) in both studies. Median time to CR was 4 months in Study 1 and 6 months in Study 2 (range 2 to 9).
| Response Rate | Study 1 (N=96) |
Study 2 (N=71) |
|---|---|---|
|
ORR (CR + PR), n (%) [95% Confidence Interval] |
33 (34) [25, 45] |
25 (35) [25, 49] |
| CR, n (%) [95% Confidence Interval] |
6 (6) [2, 13] |
4 (6) [2, 14] |
| PR, n (%) [95% Confidence Interval] |
27 (28) [19, 38] |
21 (30) [20, 43] |
| Duration of Response (months) | ||
| N | 33 | 25 |
| Median (range) | 15 (1, 20*) | 11 (1, 66*) |
| *denotes censored value | ||
14.2 Peripheral T-Cell Lymphoma
ISTODAX was evaluated in a multicenter, single-arm, international clinical study in patients with PTCL who had failed at least 1 prior systemic therapy (Study 3). Patients in US, Europe and Australia were treated with ISTODAX at a dose of 14 mg/m2 infused over 4 hours on days 1, 8, and 15 every 28 days. Of the 131 patients treated, 130 patients had histological confirmation by independent central review and were evaluable for efficacy (HC Population). Six cycles of treatment were planned; patients who developed progressive disease (PD), significant toxicity, or who met another criterion for study termination were to discontinue treatment. Responding patients had the option of continuing treatment beyond 6 cycles at the discretion of the patient and Investigator until study withdrawal criteria were met.
Primary assessment of efficacy was based on rate of complete response (CR + CRu) as determined by an Independent Review Committee (IRC) using the International Workshop Response Criteria (IWC). Secondary measures of efficacy included IRC assessment of duration of response and objective disease response (ORR, CR + CRu + PR).
Baseline Patient Characteristics
Demographic and disease characteristics of the PTCL patients are provided in Table 5.
| Characteristic | Study 3 (N=130) |
Study 4 (N=47) |
|---|---|---|
| *Stage of disease was reported at time of diagnosis for Study 3 and at time of study entry for Study 4. | ||
| Age (years), n | 130 | 47 |
| Mean (SD) | 59 (13) | 59 (13) |
| Median | 61 | 59 |
| Sex, n (%) | ||
| Male | 88 (68) | 25 (53) |
| Female | 42 (32) | 22 (47) |
| Race, n (%) | ||
| White | 116 (89) | 40 (85) |
| Black | 7 (5) | 4 (9) |
| Asian | 3 (2) | 3 (6) |
| Other | 4 (3) | 0 |
| PTCL Subtype Based on Central Diagnosis, n (%) | ||
| PTCL Unspecified (NOS) | 69 (53) | 28 (60) |
| Angioimmunoblastic T-cell lymphoma (AITL) | 27 (21) | 7 (15) |
| ALK-1 negative anaplastic large cell lymphoma (ALCL) | 21 (16) | 5 (11) |
| Other | 13 (10) | 7 (16) |
| Stage of Disease, n (%)* | ||
| I/II | 39 (30) | 2 (4) |
| III/IV | 91 (70) | 45 (96) |
| ECOG Performance Status, n (%) | ||
| 0 | 46 (35) | 20 (43) |
| 1 | 67 (51) | 22 (47) |
| 2 | 17 (13) | 4 (9) |
| Number of Prior Systemic Therapies | ||
| Median (Range) | 2 (1, 8) | 3 (1, 6) |
All patients in both studies had received prior systemic therapy for PTCL. In Study 4, a greater percentage of patients had extensive prior radiation and chemotherapy. Twenty-one patients (16%) in Study 3 and 18 patients (38%) in Study 4 had received prior autologous stem cell transplant and 31 (24%) and 19 (40%) patients, respectively, had received prior radiation therapy.
Clinical Results
Efficacy outcomes for PTCL patients as determined by the IRC are provided in Table 6 for Study 3. The complete response rate was 15% and overall response rate was 25%. Similar complete response rates were observed by the IRC across the 3 major PTCL subtypes (NOS, AITL, and ALK-1 negative ALCL). Median time to objective response was 1.8 months (~2 cycles) for the 33 patients who achieved CR, CRu or PR and was 3.7 months (~4 cycles) for the 19 patients with complete response. The responses in 11 of the 19 patients achieving CR and CRu were known to exceed 9.2 months; the follow-up on the remaining 8 patients was discontinued prior to 9.2 months.
| Response Rate | Study 3 (N=130) |
|---|---|
| 1 Primary Endpoint | |
| 2 Secondary Endpoint | |
| 3 95% Confidence Interval | |
| CR+CRu, n (%)1 | 19 (14.6) [9.0, 21.9]3 |
| PR, n (%)2 | 14 (10.8) [6.0, 17.4]3 |
| ORR (CR+CRu+PR), n (%)2 | 33 (25.4) [18.2, 33.8]3 |
In a second single-arm clinical study in patients with PTCL who had failed prior therapy (Study 4), patients were treated with ISTODAX at a starting dose of 14 mg/m2 infused over 4 hours on days 1, 8, and 15 every 28 days. Patients could be treated until disease progression at the discretion of the patient and the Investigator. The percentage of patients achieving CR + CRu in Study 4 was similar to that in Study 3.
15 REFERENCES
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. ASHP Guidelines on Handling Hazardous Drugs: Am J Health-Syst Pharm. 2006; 63:1172-1193.
- Polovich M, White JM, Kelleher LO (eds). Chemotherapy and biotherapy guidelines and recommendations for practice (2nd ed.) 2005. Pittsburgh, PA: Oncology Nursing Society.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
ISTODAX is supplied as a kit including a sterile, lyophilized powder in a single-use vial containing 10 mg of romidepsin and 20 mg of the bulking agent, povidone, USP. In addition, each kit includes one sterile Diluent vial containing 2 mL (deliverable volume) of 80% propylene glycol, USP, and 20% dehydrated alcohol, USP.
NDC 59572-983-01: ISTODAX® KIT containing 1 vial of romidepsin, 10 mg and 1 vial of diluent for romidepsin, 2 mL per carton
16.2 Storage
ISTODAX (romidepsin) for injection is supplied as a kit containing two vials in a single carton. The carton must be stored at 20° to 25°C, excursions permitted between 15° to 30°C. (See USP Controlled Room Temperature.)
Keep out of reach of children.
Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published1-4 [See References (15)].
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling.
17.1 Instructions
- Nausea and Vomiting
Nausea and vomiting are common following treatment with ISTODAX. Prophylactic antiemetics are recommended to be used in all patients. Advise patients to report these symptoms so that appropriate treatment can be instituted [See Adverse Reactions (6)].
- Low Blood Counts
Patients should be informed that treatment with ISTODAX can cause low blood counts and that frequent monitoring of hematologic parameters is required. Patients should be instructed to report fever or other signs of infection, significant fatigue, shortness of breath, or bleeding [See Warnings and Precautions (5.1)].
- Infections
Patients should be informed that infections may occur during treatment with ISTODAX. Patients should be instructed to report fever, cough, shortness of breath with or without chest pain, burning on urination, flu-like symptoms, muscle aches, or worsening skin problems [See Warnings and Precautions (5.2)].
- Tumor Lysis Syndrome
Patients at risk of tumor lysis syndrome (i.e., those with advanced stage disease and/or high tumor burden) should be monitored closely for TLS and appropriate measures taken if symptoms are observed [See Warnings and Precautions (5.4)].
- Use in Pregnancy
If pregnancy occurs during treatment with ISTODAX, female patients should be advised to seek immediate medical advice and counseling.[See Warnings and Precautions (5.5)].
- Patients should be instructed to read the patient insert carefully.
Manufactured for:
Celgene Corporation
Summit, NJ 07901
Manufactured by:
Ben Venue Laboratories, Inc.
Bedford, OH 44146
or
Baxter Oncology GmbH
Halle/Westfalen, Germany
ISTODAX® is a registered trademark of Celgene Corporation
© 2010-2013 Celgene Corporation. All Rights Reserved.
U.S. Patents: 4,977,138; 7,608,280; 7,611,724
ISTBAXPI.005/PPI.005 06/13
Patient Medication Information
ISTODAX (ISS toe dax) (romidepsin) for injection
Read the patient information that comes with ISTODAX before you receive your first treatment and each time before you are treated. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What is ISTODAX?
ISTODAX is a prescription medicine used to treat people with a type of cancer called cutaneous T-cell lymphoma (CTCL) or peripheral T-cell lymphoma (PTCL) after at least one other type of medicine by mouth or injection has been tried.
It is not known if ISTODAX is safe and effective in children under 18 years of age.
What should I tell my doctor before I receive ISTODAX?
Before receiving ISTODAX, tell your doctor if you:
- have any heart problems, including an irregular or fast heartbeat, or a condition called QT prolongation.
- have kidney problems
- have liver problems
- have problems with the amount of potassium or magnesium in your blood
- have nausea, vomiting, or diarrhea
- have any other medical conditions
- are pregnant or plan to become pregnant. ISTODAX may harm your unborn baby. Talk to your doctor about the best way to prevent pregnancy while receiving ISTODAX. Tell your doctor right away if you become pregnant while receiving ISTODAX.
- are breastfeeding or plan to breastfeed. It is not known if ISTODAX passes into your breast milk. You and your doctor should decide if you will receive ISTODAX or breast-feed. Talk to your doctor about the best way to feed your baby while you are being treated with ISTODAX.
Tell your doctor about all of the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements and any recent changes in medications.
Some medicines may affect how ISTODAX works, or ISTODAX may affect how your other medicines work. Especially tell your doctor if you take or use:
- warfarin sodium (Coumadin, Jantoven) or any other blood thinner medicine. Ask your doctor if you are not sure if you are taking a blood thinner. Your doctor may want to test your blood more often.
- a medicine to treat abnormal heart beats
- St. John's Wort (Hypericum perforatum)
- Dexamethasone (a steroid)
- Medicine for:
- tuberculosis (TB)
- seizures (epilepsy)
- bacterial infections (antibiotics)
- fungal infections (antifungals)
- HIV (AIDS)
- depression
Ask your doctor if you are not sure if your medicine is one that is listed above. Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How should I take ISTODAX?
- ISTODAX will be given to you by your doctor or nurse as an intravenous (IV) injection into your vein usually over 4 hours.
- ISTODAX is usually given on Day 1, Day 8, and Day 15 of a 28 day cycle of treatment.
- Your doctor will decide how long you will receive treatment with ISTODAX.
- Your doctor will check your blood cell counts and other blood tests regularly during your treatment with ISTODAX to check for side effects of ISTODAX. Your doctor may decide to do other tests to check your health as needed.
- Your doctor may stop your treatment, change when you get your treatment, or change the dose of your treatment if you have certain side effects while taking ISTODAX.
What are the possible side effects of ISTODAX?
ISTODAX may cause serious side effects, including:
-
Low blood cell counts: Your doctor will regularly do blood tests to check your blood counts.
- Low platelets: can cause unusual bleeding, or bruising under the skin. Talk to your doctor right away if this happens.
- Low red blood cells: may make you feel tired and you may get tired easily. You may look pale, and feel short of breath. Tell your doctor if you have these symptoms.
- Low white blood cells: can cause you to get infections, which may be serious.
-
Serious Infections. Patients receiving ISTODAX can develop serious infections that can sometimes lead to death. These infections can happen during treatment and within 30 days after treatment with ISTODAX. Your risk of infection may be higher if you have had chemotherapy in the past. Tell your doctor right away if you have any of these symptoms of infection:
- fever
- cough
- shortness of breath
with or without chest
pain
- burning with urination
- flu like symptoms
- muscle aches
- worsening skin problems
- Changes in your heartbeat. Your doctor may check your heart by doing an ECG (electrocardiogram) and your potassium and magnesium levels in your blood before you start your ISTODAX treatment. Tell your doctor if you feel an abnormal heart beat, feel dizzy or faint, have chest pain or shortness of breath. These may be symptoms related to QT prolongation and ST segment changes.
- Tumor Lysis Syndrome (TLS). TLS is a problem of the rapid breakdown of cancer cells that can happen during your treatment with ISTODAX. Your doctor may do blood tests to check for TLS and may give you medicine to prevent or treat TLS.
Common side effects of ISTODAX include:
- nausea, vomiting, diarrhea, and loss of appetite
- tiredness
Tell your doctor if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of ISTODAX. For more information, ask your doctor or pharmacist.
Ask your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about ISTODAX
Medicines are sometimes prescribed for purposes other than those listed in patient information leaflets.
This patient information leaflet summarizes the most important information about ISTODAX. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about ISTODAX that is written for health professionals. For more information, go to www.ISTODAX.com or call 1-888-423-5436.
What are the ingredients in ISTODAX?
Active ingredient: romidepsin
Inactive ingredients: povidone. The diluent contains 80% propylene glycol and 20% dehydrated alcohol.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Celgene Corporation
Summit, NJ 07901
ISTODAX® is a registered trademark of Celgene Corporation
©2010-2013 Celgene Corporation. All Rights Reserved.
U.S. Patents: 4,977,138; 7,608,280; 7,611,724
ISTBAXPPI.005 06/13
Approved June/2013
PRINCIPAL DISPLAY PANEL - Kit Carton - 10 mg/vial

PRINCIPAL DISPLAY PANEL - Istodax - 10 mg/vial
PRINCIPAL DISPLAY PANEL - Sterile Diluent - 2 mL per single use vial
ISTODAXromidepsin KIT
| ||||||||||||||||||||||||||||||||||||||||