J-TAN
JayMac Pharmaceuticals LLC
Great Southern Laboratories
J-TAN PD Drops
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts
- J-TAN Uses
- Warnings
- Directions
- J-TAN Other information
- Inactive ingredients
- Questions? Comments?
- Product Packaging
FULL PRESCRIBING INFORMATION
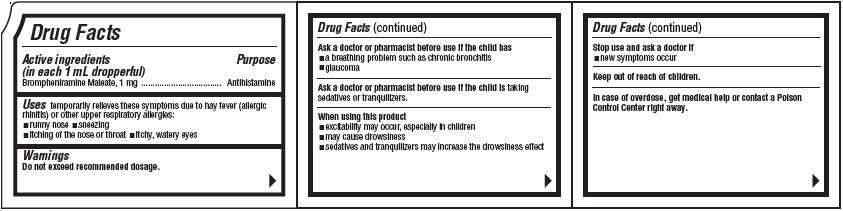
Drug Facts
Acitive ingredients(in each 1 mL dropperful)
Purpose
J-TAN Uses
- runny nose
- sneezing
- itching of the nose or throat
- itchy, water eyes
Warnings
Do not exceed recommended dosage.Ask a doctor or pharmacist before use if the child has
- a breathing problem such as chronic bronchitis
- glaucoma
When using this product
- excitability may occur, especially in children
- may cause drowsiness
- sedatives and tranquilizers may increase the drowsiness effect
Stop use and ask a doctor if
- new symptoms occur
Keep out of reach of children.
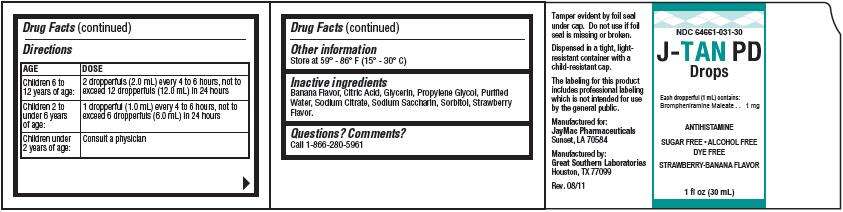
In case of overdose, get medical help or contact a Poison Control Center right away.Directions
| AGE | DOSE |
| Children 6 to 12 years of age: |
2 dropperfuls (2.0 mL) every 4 to 6 hours, not to exceed 12 dropperfuls (12.0 mL) in 24 hours |
| Children 2 to under 6 years of age: |
1 dropperful (2.0 mL) every 4 to 6 hours, not to exceed 6 dropperfuls (6.0 mL) in 24 hours |
| Children under 2 years of age: |
Consult a physician |
J-TAN Other information
Inactive ingredients
Questions? Comments?
Product Packaging
NDC 64661-031-30
J-TAN PD
Drops
Each dropperful (1 mL) contains:
ANTIHISTAMINE
SUGAR FREE·ALCOHOL FREE
DYE FREE
STRAWBERRY-BANANA FLAVOR
1 fl oz (30 mL)
JayMac Pharmaceuticals
Great Southern Laboratories

J-TANBrompheniramine Maleate LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!