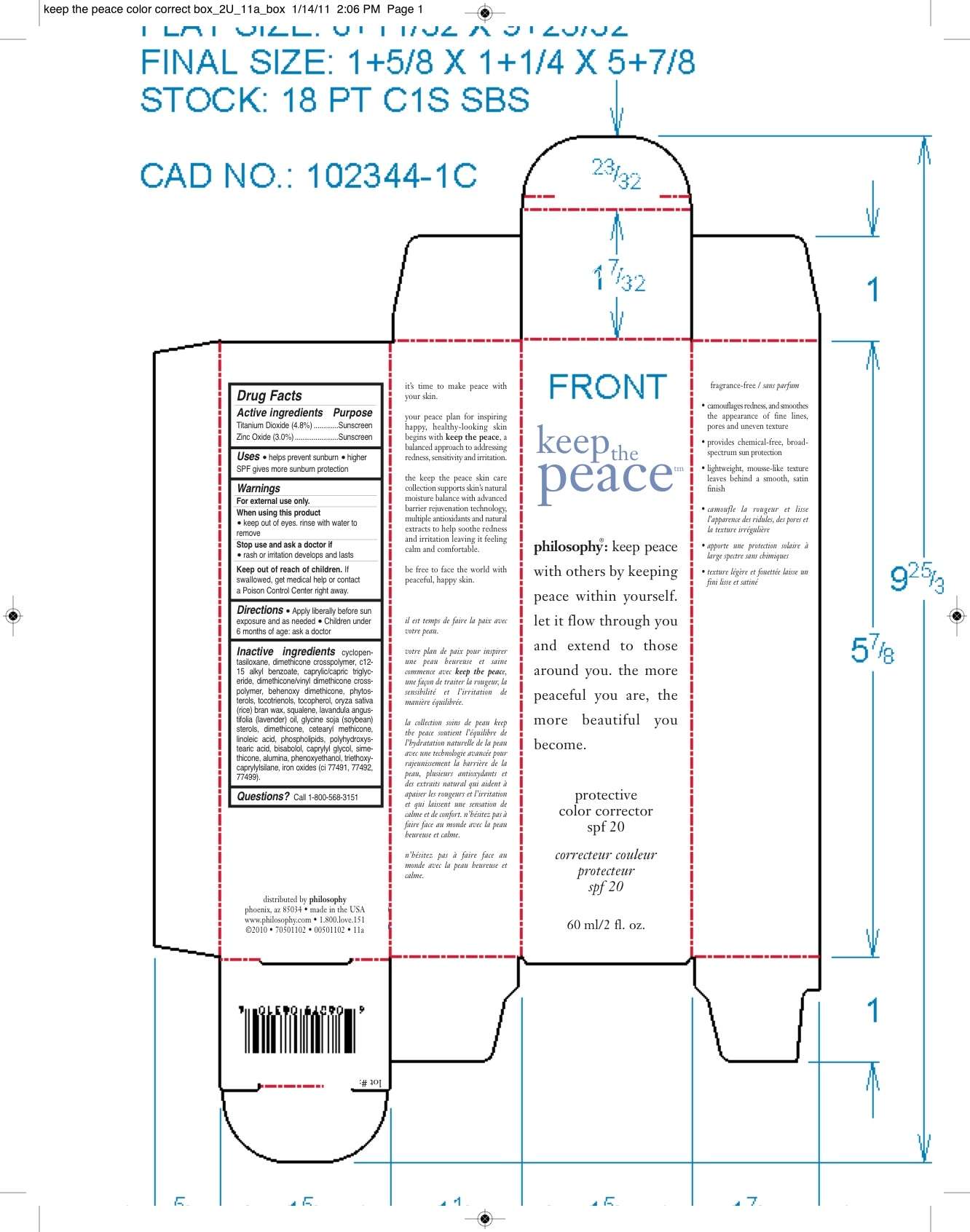
Keep The Peace Protect and Correct
DRUG FACTS
FULL PRESCRIBING INFORMATION
Active ingredient
TITANIUM DIOXIDE......4.8%
ZINC OXIDE..........3.0%
CYCLOPENTASILOXANE, DIMETHICONE CROSSPOLYMER, C12-15 ALKYL BENZOATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, BEHENOXY DIMETHICONE, PHYTOSTEROLS, TOCOTRIENOLS, TOCOPHEROL, ORYZA SATIVA (RICE) BRAN WAX, SQUALENE, LAVANDULA ANGUSTIFOLIA (LAVENDER) OIL, GLYCINE SOJA (SOYBEAN) STEROLS, DIMETHICONE, CETEARYL METHICONE, LINOLEIC ACID, PHOSPHOLIPIDS, POLYHYDROXYSTEARIC ACID, BISABOLOL, CAPYLYL GLYCOL, SIMETHICONE, ALUMINA, PHENOXYETHANOL, TRIETHOXYCAPRYLYLSILANE, IRON OXIDES (CI 77491, 77492, 77499).
Purpose
Sunscreen
Uses: helps prevent suburn
higher SPF gives more sunburn protection
FOR EXTERNAL USE ONLY.
WHEN USING THIS PRODUCT.
KEEP OUT OF EYES. RINSE WITH WATER TO REMOVE
STOP USE AND ASK DOCTOR IF A RASH OR IRRITATION DEVELOPS AND LASTS.
KEEP OUT OF REACH OF CHILDREN.
IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
QUESTIONS? CALL - 1-800-568-3515
Keep The Peace Protect and CorrectTitanium Dioxide and Zinc Oxide CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||