KETALAR
General Injectables & Vaccines, Inc
Ketalar®(Ketamine Hydrochloride Injection, USP)CIII
FULL PRESCRIBING INFORMATION: CONTENTS*
- SPECIAL NOTE
- KETALAR DESCRIPTION
- CLINICAL PHARMACOLOGY
- KETALAR INDICATIONS AND USAGE
- KETALAR CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- KETALAR ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- KETALAR DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- ANIMAL PHARMACOLOGY AND TOXICOLOGY
- SAMPLE PACKAGE LABEL
FULL PRESCRIBING INFORMATION
SPECIAL NOTE
EMERGENCE REACTIONS HAVE OCCURRED IN APPROXIMATELY 12 PERCENT OF PATIENTS.
THE PSYCHOLOGICAL MANIFESTATIONS VARY IN SEVERITY BETWEEN PLEASANT DREAM-LIKE STATES, VIVID IMAGERY, HALLUCINATIONS, AND EMERGENCE DELIRIUM. IN SOME CASES THESE STATES HAVE BEEN ACCOMPANIED BY CONFUSION, EXCITEMENT, AND IRRATIONAL BEHAVIOR WHICH A FEW PATIENTS RECALL AS AN UNPLEASANT EXPERIENCE. THE DURATION ORDINARILY IS NO MORE THAN A FEW HOURS; IN A FEW CASES, HOWEVER, RECURRENCES HAVE TAKEN PLACE UP TO 24 HOURS POSTOPERATIVELY. NO RESIDUAL PSYCHOLOGICAL EFFECTS ARE KNOWN TO HAVE RESULTED FROM USE OF KETALAR.
THE INCIDENCE OF THESE EMERGENCE PHENOMENA IS LEAST IN THE ELDERLY (OVER 65 YEARS OF AGE) PATIENT. ALSO, THEY ARE LESS FREQUENT WHEN THE DRUG IS GIVEN INTRAMUSCULARLY AND THE INCIDENCE IS REDUCED AS EXPERIENCE WITH THE DRUG IS GAINED.
THE INCIDENCE OF PSYCHOLOGICAL MANIFESTATIONS DURING EMERGENCE, PARTICULARLY DREAM-LIKE OBSERVATIONS AND EMERGENCE DELIRIUM, MAY BE REDUCED BY USING LOWER RECOMMENDED DOSAGES OF KETALAR IN CONJUCTION WITH INTRAVENOUS DIAZEPAM DURING INDUCTION AND MAINTENANCE OF ANESTHESIA. (See DOSAGE AND ADMINISTRATION Section.) ALSO, THESE REACTIONS MAY BE REDUCED IF TACTILE, AND VISUAL STIMULATION OF THE PATIENT IS MINIMIZED DURING THE RECOVERY PERIOD. THIS DOES NOT PRECLUDE THE MONITORING OF VITAL SIGNS.
IN ORDER TO TERMINATE A SEVERE EMERGENCE REACTION, THE USE OF A SMALL HYPNOTIC DOSE OF A SHORT-ACTING OR ULTRA SHORT-ACTING BARBITURATE MAY BE REQUIRED.
WHEN KETALAR IS USED ON AN OUTPATIENT BASIS, THE PATIENT SHOULD NOT BE RELEASED UNTIL RECOVERY FROM ANESTHESIA IS COMPLETE AND THEN SHOULD BE ACCOMPANIED BY A RESPONSIBLE ADULT.
KETALAR DESCRIPTION
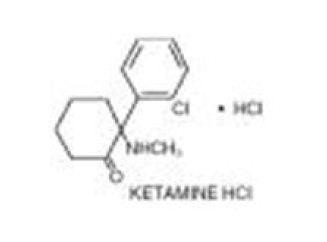
Ketalar is a nonbarbiturate anesthetic chemically designated dl 2-(0-chlorophenyl)-2-(methylamino) cyclohexanone hydrochloride. It is formulated as a slightly acid (pH 3.5-5.5) sterile solution for intravenous or intramuscular injection in concentrations containing the equivalent of either 10, 50 or 100 mg ketamine base per milliliter and contains not more than 0.1 mg/mL Phemerol® (benzethonium chloride) added as a preservative. The 10 mg/mL solution has been made isotonic with sodium chloride.
CLINICAL PHARMACOLOGY
Ketalar is a rapid-acting general anesthetic producing an anesthetic state characterized by profound analgesia, normal pharyngeal-laryngeal reflexes, normal or slightly enhanced skeletal muscle tone, cardiovascular and respiratory stimulation, and occasionally a transient and minimal respiratory depression.
A patent airway is maintained partly by virtue of unimpaired pharyngeal and laryngeal reflexes. (See WARNINGS and PRECAUTIONS Sections.)
The biotransformation of Ketalar includes N-dealkylation (metabolite I), hydroxylation of the cyclohexone ring (metabolites III and IV), conjugation with glucuronic acid and dehydration of the hydroxylated metabolites to form the cyclohexene derivative (metabolite II).
Following intravenous administration, the ketamine concentration has an initial slope (alpha phase) lasting about 45 minutes with a half-life of 10 to 15 minutes. The first phase corresponds clinically to the anesthetic effect of the drug. The anesthetic action is terminated by a combination of redistribution from the CNS to slower equilibrating peripheral tissues and by hepatic biotransformation to metabolite I. This metabolite is about 1/3 as active as ketamine in reducing halothane requirements (MAC) of the rat. The later half-life of ketamine (beta phase) is 2.5 hours.
The anesthetic state produced by Ketalar has been termed "dissociative anesthesia" in that it appears to selectively interrupt association pathyways of the brain before producing somatesthetic sensory blockade. It may selectively depress the thalamoneocortical system before significantly obtunding the more ancient cerebral centers and pathways (reticular-activating and limbic systems).
Elevation of blood pressure begins shortly after injection, reaches a maximum within a few minutes and usually returns to preanesthetic values within 15 minutes after injection. In the majority of cases, the systolic and diastolic blood pressure peaks from 10% to 50% above preanesthetic levels shortly after induction of anesthesia, but the elevation can be higher or longer in individual cases (see CONTRAINDICATIONS Section).
Ketamine has a wide margin of safety; several instances of unintentional administration of overdoses of Ketalar (up to ten times that usually required) have been followed by prolonged but complete recovery.
Ketalar has been studied in over 12,000 operative and diagnostic procedures, involving over 10,000 patients from 105 separate studies. During the course of these studies Ketalar was administered as the sole agent, as induction for other general agents, or to supplement low-potency agents.
Specific areas of application have included the following:
- debridement, painful dressings, and skin grafting in burn patients, as well as other superficial surgical procedures.
- neurodiagnostic procedures such as pneumonencephalograms, ventriculograms, myelograms, and lumbar punctures. See also Precaution concerning increased intracranial pressure.
- diagnostic and operative procedures of the eye, ear, nose, and mouth, including dental extractions.
- diagnostic and operative procedures of the pharynx, larynx, or bronchial tree. NOTE: Muscle relaxants, with proper attention to respiration, may be required (see PRECAUTIONS Section).
- sigmoidoscopy and minor surgery of the anus and rectum, and circumcision.
- extraperitoneal procedures used in gynecology such as dilatation and curettage.
- orthopedic procedures such as closed reductions, manipulations, femoral pinning, amputations, and biopsies.
- as an anesthetic in poor-risk patients with depression of vital functions.
- in procedures where the intramuscular route of administration is preferred.
- in cardiac catheterization procedures.
In these studies, the anesthesia was rated either "excellent" or "good" by the anesthesiologist and the surgeon at 90% and 93%, respectively; rated "fair" at 6% and 4%, respectively; and rated "poor" at 4% and 3%, respectively. In a second method of evaluation, the anesthesia was rated "adequate" in at least 90%, and "inadequate" in 10% or less of the procedures.
KETALAR INDICATIONS AND USAGE
Ketalar is indicated as the sole anesthetic agent for diagnostic and surgical procedures that do not require skeletal muscle relaxation. Ketalar is best suited for short procedures but it can be used, with additional doses, for longer procedures.
Ketalar is indicated for the induction of anesthesia prior to the administration of other general anesthetic agents.
Ketalar is indicated to supplement low-potency agents, such as nitrous oxide.
Specific areas of application are described in the CLINICAL PHARMACOLOGY Section.
KETALAR CONTRAINDICATIONS
Ketamine hydrochloride is contraindicated in those in whom a significant elevation of blood pressure would constitute a serious hazard and in those who have shown hypersensitivity to the drug.
WARNINGS
Cardiac function should be continually monitored during the procedure in patients found to have hypertension or cardiac decompensation.
Postoperative confusional states may occur during the recovery period. (See Special Note.)
Respiratory depression may occur with overdosage or too rapid a route of administration of Ketalar, in which case supportive ventilation should be employed. Mechanical support of respiration is preferred to administration of analeptics.
PRECAUTIONS
General
Ketalar should be used by or under the direction of physicians experienced in administering general anesthetics and in maintenance of an airway and in the control of respiration.
Because pharyngeal and laryngeal reflexes are usually active, Ketalar should not be used alone surgery or diagnostic procedures of the pharynx, larynx, or bronchial tree. Mechanical stimulation of the pharynx should be avoided, whenever possible, if Ketalar is used alone. Muscle relaxants, with proper attention to respiration, may be required in both of these instances.
Resuscitative equipment should be ready for use.
The incidence of emergence reactions may be reduced if verbal and tactile stimulation of the patient is minimized during the recovery period. This does not preclude the monitoring of vital signs (see Special Note).
The intravenous dose should be administered over a period of 60 seconds. More rapid administration may result in respiratory depression or apnea and enhanced pressor response.
In surgical procedures involving visceral pain pathways, Ketalar should be supplemented with an agent which obtunds visceral pain.
Use with caution in the chronic alcoholic and the acutely alcohol-intoxicated patient.
An increase in cerebrospinal fluid pressure has been reported following administration of ketamine hydrochloride. Use with extreme caution in patients with preanesthetic elevated cerebrospinal fluid pressure.
Information for Patients
As appropriate, especially in cases where early discharge is possible, the duration of Ketalar and other drugs employed during the conduct of anesthesia should be considered. The patients should be cautioned that driving an automobile, operating hazardous machinery, or engaging in hazardous activities should not be undertaken for 24 hours or more (depending upon the dosage of Ketalar and consideration of other drugs employed) after anesthesia.
Drug Interactions
Prolonged recovery time may occur if barbiturates and/or narcotics are used concurrently with Ketalar.
Ketalar is clinically compatible with the commonly used general and local anesthetic agents when an adequate respiratory exchange is maintained.
Usage in Pregnancy
Since the safe use in pregnancy, including obstetrics (either vaginal or abdominal delivery), has not been established, such use is not recommended (see ANIMAL PHARMACOLOGY AND TOXICOLOGY , Reproduction ).
Geriatric Use
Clinical studies of ketamine hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 16 have not been established.
KETALAR ADVERSE REACTIONS
Cardiovascular: Blood pressure and pulse rate are frequently elevated following administration of Ketalar alone. However, hypotension and bradycardia have been observed. Arrhythmia has also occurred.
Respiration: Although respiration is frequently stimulated, severe depression of respiration or apnea may occur following rapid intravenous administration of high doses of Ketalar. Laryngospasms and other forms of airway obstruction have occurred during Ketalar anesthesia.
Eye: Diplopia and nystagmus have been noted following Ketalar administration. It also may cause a slight elevation in intraocular pressure measurement.
Psychological: (See Special Note.)
Neurological: In some patients, enhanced skeletal muscle tone may be manifested by tonic and clonic movements sometimes resembling seizures (see DOSAGE AND ADMINISTRATION Section).
Gastrointestinal: Anorexia, nausea and vomiting have been observed; however, this is not usually severe and allows the great majority of patients to take liquids by mouth shortly after regaining consciousness (see DOSAGE AND ADMINISTRATION Section).
General: Anaphylaxis. Local pain and exanthema at the injection site have infrequently been reported. Transient erythema and/or morbilliform rash have also been reported.
For medical advice about adverse reactions contact your medical professional. To report SUSPECTED ADVERSE REACTIONS, contact JHP at 1-866-923-2547 or MEDWATCH at 1-800-FDA-1088 (1-800-332-1088) or http://www.fda.gov/medwatch/.
DRUG ABUSE AND DEPENDENCE
Ketamine has been reported being used as a drug of abuse. Reports suggest that ketamine produces a variety of symptoms including, but not limited to anxiety, dysphoria, disorientation, insomnia, flashbacks, hallucinations, and psychotic episodes. Severe irritative and inflammatory urinary tract and bladder symptoms including cystitis have been reported in individuals with history of chronic ketamine use or abuse. Ketamine dependence and tolerance are possible following prolonged administration. A withdrawal syndrome with psychotic features has been described following discontinuation of long-term ketamine use. Therefore, ketamine should be prescribed and administered with caution.
OVERDOSAGE
Respiratory depression may occur with overdosage or too rapid a rate of administration of Ketalar, in which case supportive ventilation should be employed. Mechanical support of respiration is preferred to administration of analeptics.
KETALAR DOSAGE AND ADMINISTRATION
Note: Barbiturates and Ketalar, being chemically incompatible because of precipitate formation, should not be injected from the same syringe.
If the ketalar dose is augmented with diazepam, the two drugs must be given separately. Do not mix Ketalar and diazepam in syringe or infusion flask. For additional information on the use of diazepam, refer to the WARNINGS and DOSAGE AND ADMINISTRATION Sections of the diazepam insert.
Preoperative Preparations:
- While vomiting has been reported following Ketalar administration, some airway protection may be afforded because of active laryngeal-pharyngeal reflexes. However, since aspiration may occur with Ketalar and since protective reflexes may also be diminished by supplementary anesthetics and muscle relaxants, the possibility of aspiration must be considered. Ketalar is recommended for use in the patient whose stomach is not empty when, in the judgment of the practitioner, the benefits of the drug outweigh the possible risks.
- Atropine, scopolamine, or another drying agent should be given at an appropriate interval prior to induction.
Onset and Duration:
Because of rapid induction following the initial intravenous injection, the patient should be in a supported position during administration.
The onset of action of Ketalar is rapid; an intravenous dose of 2 mg/kg (1 mg/lb) of body weight usually produces surgical anesthesia within 30 seconds after injection, with the anesthetic effect usually lasting five to ten minutes. If a longer effect is desired, additional increments can be administered intravenously or intramuscularly to maintain anesthesia without producing significant cumulative effects.
Intramuscular doses, in a range of 9 to 13 mg/kg (4 to 6 mg/lb) usually produce surgical anesthesia within 3 to 4 minutes following injection, with the anesthetic effect usually lasting 12 to 25 minutes.
Dosage:
As with other general anesthetic agents, the individual response to Ketalar is somewhat varied depending on the dose, route of administration, and age of patient, so that dosage recommendation cannot be absolutely fixed. The drug should be titrated against the patient's requirements.
Induction:
Intravenous Route: The initial dose of Ketalar administered intravenously may range from 1 mg/kg to 4.5 mg/kg (0.5 to 2 mg/lb). The average amount required to produce five to ten minutes of surgical anesthesia has been 2 mg/kg (1 mg/lb).
Alternatively, in adult patients an induction dose of 1 mg to 2 mg/kg intravenous ketamine at a rate of 0.5 mg/kg/min may be used for induction of anesthesia. In addition, diazepam in 2 mg to 5 mg doses, administered in a separate syringe over 60 seconds, may be used. In most cases, 15 mg of intravenous diazepam or less will suffice. The incidence of psychological manifestations during emergence, particularly dream-like observations and emergence delirium, may be reduced by this induction dosage program.
Note: The 100 mg/mL concentration of Ketalar should not be injected intravenously without proper dilution. It is recommended the drug be diluted with an equal volume of either Sterile Water for injection, USP, Normal Saline, or 5% Dextrose in Water.
Rate of Administration: It is recommended that Ketalar be administered slowly (over a period of 60 seconds). More rapid administration may result in respiratory depression and enhanced pressor response.
Intramuscular Route: The initial dose of Ketalar administered intramuscularly may range from 6.5 to 13 mg/kg (3 to 6 mg/lb). A dose of 10 mg/kg (5 mg/lb) will usually produce 12 to 25 minutes of surgical anesthesia.
Maintenance of Anesthesia:
The maintenance dose should be adjusted according to the patient's anesthetic needs and whether an additional anesthetic agent is employed.
Increments of one-half to the full induction dose may be repeated as needed for maintenance of anesthesia. However, it should be noted that purposeless and tonic-clonic movements of extremities may occur during the course of anesthesia. These movements do not imply a light plane and are not indicative of the need for additional doses of the anesthetic.
It should be recognized that the larger the total dose of Ketalar administered, the longer will be the time to complete recovery.
Adult patients induced with Ketalar augmented with intravenous diazepam may be maintained on Ketalar given by slow microdrip infusion technique at a dose of 0.1 to 0.5 mg/minute, augmented with diazepam 2 to 5 mg administered intravenously as needed. In many cases 20 mg or less of intravenous diazepam total for combined induction and maintenance will suffice. However, slightly more diazepam may be required depending on the nature and duration of the operation, physical status of patient, and other factors. The incidence of psychological manifestations during emergence, particularly dream-like observations and emergence delirium, may be reduced by this maintenance dosage program.
Dilution: To prepare a dilute solution containing 1 mg of ketamine per mL, aseptically transfer 10 mL (50 mg per mL) or 5 mL (100 mg per mL) to 500 mL of 5% Dextrose Injection, USP or Sodium Chloride (0.9%) Injection, USP (Normal Saline) and mix well. The resultant solution will contain 1 mg of ketamine per mL.
The fluid requirements of the patient and duration of anesthesia must be considered when selecting the appropriate dilution of Ketalar. If fluid restriction is required, Ketalar can be added to a 250 mL infusion as described above to provide a Ketalar concentration of 2 mg/mL.
Ketalar 10 mg/mL are not recommended for dilution.
Supplementary Agents:
Ketalar is clinically compatible with the commonly used general and local anesthetic agents when an adequate respiratory exchange is maintained.
The regimen of a reduced dose of Ketalar supplemented with diazepam can be used to produce balanced anesthesia by combination with other agents such as nitrous oxide and oxygen.
HOW SUPPLIED
Ketalar is supplied as the hydrochloride in concentrations equivalent to ketamine base.
NDC 42023-113-10 - Each 20-mL multi-dose vial contains 10 mg/mL. Supplied in cartons of 10.
NDC 42023-114-10 - Each 10-mL multi-dose vial contains 50 mg/mL. Supplied in cartons of 10.
NDC 42023-115-10 - Each 5-mL multi-dose vial contains 100 mg/mL. Supplied in cartons of 10.
Store at 20°-25°C (68°-77°F). (See USP controlled room temperature.)
Protect from light.
Rx only.
ANIMAL PHARMACOLOGY AND TOXICOLOGY
Toxicity: The acute toxicity of Ketalar has been studied in several species. In mature mice and rats, the intraperitoneal LD50 values are approximately 100 times the average human intravenous dose and approximately 20 times the average human intramuscular dose. A slightly higher acute toxicity observed in neonatal rats was not sufficiently elevated to suggest an increased hazard when used in pediatric patients. Daily intravenous injections in rats of five times the average human intravenous dose and intramuscular injections in dogs at four times the average human intramuscular dose demonstrated excellent tolerance for as long as 6 weeks. Similarly, twice weekly anesthetic sessions of one, three, or six hours' duration in monkeys over a four- to six-week period were well tolerated.
Interaction with Other Drugs Commonly Used for Preanesthetic Medication: Large doses (three or more times the equivalent effective human dose) of morphine, meperidine, and atropine increased the depth and prolonged the duration of anesthesia produced by a standard anesthetizing dose of Ketalar in Rhesus monkeys. The prolonged duration was not of sufficient magnitude to contraindicate the use of these drugs for preanesthetic medication in human clinical trials.
Blood Pressure: Blood pressure responses to Ketalar vary with the laboratory species and experimental conditions. Blood pressure is increased in normotensive and renal hypertensive rats with and without adrenalectomy and under pentobarbital anesthesia.
Intravenous Ketalar produces a fall in arterial blood pressure in the Rhesus monkey and a rise in arterial blood pressure in the dog. In this respect the dog mimics the cardiovascular effect observed in man. The pressor response to Ketalar injected into intact, unanesthetized dogs is accompanied by a tachycardia, rise in cardiac output and a fall in total peripheral resistance. It causes a fall in perfusion pressure following a large dose injected into an artificially perfused vascular bed (dog hindquarters), and it has little or no potentiating effect upon vasoconstriction responses of epinephrine or norepineprhine. The pressor response to Ketalar is reduced or blocked by chlorpromazine (central depressant and peripheral a-adrenergic blockade), by b-adrenergic blockade, and by ganglionic blockade. The tachycardia and increase in myocardial contractile force seen in intact animals does not appear in isolated hearts (Langendorff) at a concentration of 0.1 mg of Ketalar or Starling dog heart-lung preparations at a Ketalar concentration of 50 mg/kg of HLP. These observations support the hypothesis that the hypertension produced by Ketalar is due to selective activation of central cardiac stimulating mechanisms leading to an increase in cardiac output. The dog myocardium is not sensitized to epinephrine and Ketalar appears to have a weak antiarrhythmic activity.
Metabolic Disposition: Ketalar is rapidly absorbed following parenteral administration. Animal experiments indicated that Ketalar was rapidly distributed into body tissues, with relatively high concentrations appearing in body fat, liver, lung, and brain; lower concentrations were found in the heart, skeletal muscle, and blood plasma. Placental transfer of the drug was found to occur in pregnant dogs and monkeys. No significant degree of binding to serum albumin was found with Ketalar.
Balance studies in rats, dogs, and monkeys resulted in the recovery of 85% to 95% of the dose in the urine, mainly in the form of degradation products. Small amounts of drug were also excreted in the bile and feces. Balance studies with tritium-labeled Ketalar in human subjects (1 mg/lb given intravenously) resulted in the mean recovery of 91% of the dose in the urine and 3% in the feces. Peak plasma levels averaged about 0.75 ug/mL, and CSF levels were about 0.2 ug/mL, 1 hour after dosing.
Ketalar undergoes N-demethylation and hydroxylation of the cyclohexanone ring, with the formation of water-soluble conjugates which are excreted in the urine. Further oxidation also occurs with the formation of a cyclohexanone derivative. The unconjugated N-demethylated metabolite was found to be less than one-sixth as potent as Ketalar. The unconjugated demethyl cyclohexanone derivative was found to be less than one-tenth as potent as Ketalar. Repeated doses of Ketalar administered to animals did not produce any detectable increase in microsomal enzyme activity.
Reproduction: Male and female rats, when given five times the average human intravenous dose of Ketalar for three-consecutive days about one week before mating, had a reproductive performance equivalent to that of saline-injected controls. When given to pregnant rats and rabbits intramuscularly at twice the average human intramuscular dose during the respective periods of organogenesis, the litter characteristics were equivalent to those of saline-injected controls. A small group of rabbits was given a single large dose (six times the average human dose) of Ketalar on Day 6 of pregnancy to stimulate the effect of an excessive clinical dose around the period of nidation. The outcome of pregnancy was equivalent in control and treated groups.
To determine the effect of Ketalar on the perinatal and postnatal period, pregnant rats were given twice the average human intramuscular dose during Days 18 and 21 of pregnancy. Litter characteristics at birth and through the weaning period were equivalent to those of the control animals. There was a slight increase in incidence of delayed parturition by one day in treated dams of this group. Three groups each of mated beagle bitches were given 2.5 times the average human intramuscular dose twice weekly for the three weeks of the first, second, and third trimesters of pregnancy, respectively, without the development of adverse effects in the pups.
Prescribing Information as of June 2011.
JHP Pharmaceuticals
Manufactured and Distributed by:
JHP Pharmaceuticals, LLC
Rochester, MI 48307
SAMPLE PACKAGE LABEL

KETALARketamine hydrochloride INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||