Ketorolac Tromethamine
KETOROLAC TROMETHAMINE TABLETS USP, 10 mg0314Rx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- KETOROLAC TROMETHAMINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- KETOROLAC TROMETHAMINE INDICATIONS AND USAGE
- KETOROLAC TROMETHAMINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- KETOROLAC TROMETHAMINE ADVERSE REACTIONS
- OVERDOSAGE
- KETOROLAC TROMETHAMINE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
WARNING
Ketorolac tromethamine tablets, a non-steroidal anti-inflammatory drug (NSAID), are indicated for the short-term (up to 5 days in adults), management of moderately severe acute pain that requires analgesia at the opioid level and only as continuation treatment following IV or IM dosing of ketorolac tromethamine, if necessary. The total combined duration of use of ketorolac tromethamine tablets and ketorolac tromethamine should not exceed 5 days.
Ketorolac tromethamine tablets are not indicated for use in pediatric patients and they are NOT indicated for minor or chronic painful conditions. Increasing the dose of ketorolac tromethamine tablets beyond a daily maximum of 40 mg in adults will not provide better efficacy but will increase the risk of developing serious adverse events.
GASTROINTESTINAL RISK
- Ketorolac tromethamine, including ketorolac tromethamine tablets can cause peptic ulcers, gastrointestinal bleeding and/or perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Therefore, ketorolac tromethamine is CONTRAINDICATED in patients with active peptic ulcer disease, in patients with recent gastrointestinal bleeding or perforation, and in patients with a history of peptic ulcer disease or gastrointestinal bleeding. Elderly patients are at greater risk for serious gastrointestinal events (see WARNINGS ).
CARDIOVASCULAR RISK
- NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk (see WARNINGS and CLINICAL STUDIES ).
- Ketorolac tromethamine is CONTRAINDICATED for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS ).
RENAL RISK
- Ketorolac tromethamine is CONTRAINDICATED in patients with advanced renal impairment and in patients at risk for renal failure due to volume depletion (see WARNINGS ).
RISK OF BLEEDING
- Ketorolac tromethamine inhibits platelet function and is, therefore, CONTRAINDICATED in patients with suspected or confirmed cerebrovascular bleeding, patients with hemorrhagic diathesis, incomplete hemostasis and those at high risk of bleeding (see WARNINGS and PRECAUTIONS ).
Ketorolac tromethamine is CONTRAINDICATED as prophylactic analgesic before any major surgery.
RISK DURING LABOR AND DELIVERY
- The use of ketorolac tromethamine in labor and delivery is contraindicated because it may adversely affect fetal circulation and inhibit uterine contractions.
CONCOMITANT USE WITH NSAIDs
- Ketorolac tromethamine is CONTRAINDICATED in patients currently receiving aspirin or NSAIDs because of the cumulative risk of inducing serious NSAID-related side effects.
SPECIAL POPULATIONS
- Dosage should be adjusted for patients 65 years or older, for patients under 50 kg (110 lbs) of body weight (see DOSAGE AND ADMINISTRATION ) and for patients with moderately elevated serum creatinine (see WARNINGS ).
KETOROLAC TROMETHAMINE DESCRIPTION
Ketorolac Tromethamine Tablets USP are a member of the pyrrolo-pyrrole group of non-steroidal anti-inflammatory drugs (NSAIDs). The chemical name for ketorolac tromethamine, USP is (±)-5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1). The structural formula is:
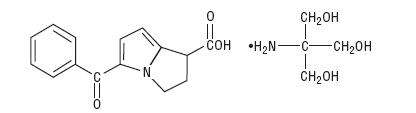
C15H13NO3 . C4H11NO3 M.W. 376.40
Ketorolac tromethamine, USP is a racemic mixture of [-]S and [+]R ketorolac tromethamine, USP. Ketorolac tromethamine, USP may exist in three crystal forms. All forms are equally soluble in water. Ketorolac tromethamine, USP has a pKa of 3.5 and an n-octanol/water partition coefficient of 0.26.
Ketorolac Tromethamine Tablets USP are white, round, convex, unscored, film coated tablets. Each tablet, for oral administration, contains 10 mg ketorolac tromethamine, USP, the active ingredient. In addition, each tablet contains the following inactive ingredients: hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, and titanium dioxide.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Ketorolac tromethamine is a non-steroidal anti-inflammatory drug (NSAID) that exhibits analgesic activity in animal models. The mechanism of action of ketorolac, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition. The biological activity of ketorolac tromethamine is associated with the S-form. Ketorolac tromethamine possesses no sedative or anxiolytic properties.
The peak analgesic effect of ketorolac tromethamine occurs within 2 to 3 hours and is not statistically significantly different over the recommended dosage range of ketorolac tromethamine. The greatest difference between large and small doses of ketorolac tromethamine is in the duration of analgesia.
Pharmacokinetics
Ketorolac tromethamine is a racemic mixture of [-]S- and [+]R-enantiomeric forms, with the S-form having analgesic activity.
Comparison of IV, IM and Oral Pharmacokinetics
The pharmacokinetics of ketorolac tromethamine, following IV and IM doses of ketorolac tromethamine and oral doses of ketorolac tromethamine, are compared in Table 1. In adults, the extent of bioavailability following administration of the ORAL form of ketorolac tromethamine and the IM form of ketorolac tromethamine was equal to that following an IV bolus.
Linear Kinetics
In adults, following administration of single ORAL doses of ketorolac tromethamine or IM or IV doses of ketorolac tromethamine in the recommended dosage ranges, the clearance of the racemate does not change. This implies that the pharmacokinetics of ketorolac tromethamine in adults, following single or multiple IM or IV doses of ketorolac tromethamine or recommended oral doses of ketorolac tromethamine, are linear. At the higher recommended doses, there is a proportional increase in the concentrations of free and bound racemate.
Absorption
Ketorolac tromethamine is 100% absorbed after oral administration (see Table 1). Oral administration of ketorolac tromethamine after a high-fat meal resulted in decreased peak and delayed time-to-peak concentrations of ketorolac tromethamine by about 1 hour. Antacids did not affect the extent of absorption.
Distribution
The mean apparent volume (Vß) of ketorolac tromethamine following complete distribution was approximately 13 liters. This parameter was determined from single-dose data. The ketorolac tromethamine racemate has been shown to be highly protein bound (99%). Nevertheless, plasma concentrations as high as 10 mcg/mL will only occupy approximately 5% of the albumin binding sites. Thus, the unbound fraction for each enantiomer will be constant over the therapeutic range. A decrease in serum albumin, however, will result in increased free drug concentrations.
Ketorolac tromethamine is excreted in human milk (see PRECAUTIONS, Nursing Mothers ).
Metabolism
Ketorolac tromethamine is largely metabolized in the liver. The metabolic products are hydroxylated and conjugated forms of the parent drug. The products of metabolism, and some unchanged drug, are excreted in the urine.
Excretion
The principal route of elimination of ketorolac and its metabolites is renal. About 92% of a given dose is found in the urine, approximately 40% as metabolites and 60% as unchanged ketorolac. Approximately 6% of a dose is excreted in the feces. A single-dose study with 10 mg ketorolac tromethamine (n = 9) demonstrated that the S-enantiomer is cleared approximately two times faster than the R-enantiomer and that the clearance was independent of the route of administration. This means that the ratio of S/R plasma concentrations decreases with time after each dose. There is little or no inversion of the R- to S- form in humans. The clearance of the racemate in normal subjects, elderly individuals and in hepatically and renally impaired patients is outlined in Table 2 (see CLINICAL PHARMACOLOGY, Kinetics in Special Populations ).
The half-life of the ketorolac tromethamine S-enantiomer was approximately 2.5 hours (SD ± 0.4) compared with 5 hours (SD ± 1.7) for the R-enantiomer. In other studies, the half-life for the racemate has been reported to lie within the range of 5 to 6 hours.
Accumulation
Ketorolac tromethamine administered as an IV bolus every 6 hours for 5 days to healthy subjects (n = 13), showed no significant difference in Cmax on Day 1 and Day 5. Trough levels averaged 0.29 mcg/mL (SD ± 0.13) on Day 1 and 0.55 mcg/mL (SD ± 0.23) on Day 6. Steady state was approached after the fourth dose.
Accumulation of ketorolac tromethamine has not been studied in special populations (geriatric, pediatric, renal failure or hepatic disease patients).
Kinetics in Special Populations
Geriatric Patients
Based on single-dose data only, the half-life of the ketorolac tromethamine racemate increased from 5 to 7 hours in the elderly (65 to 78 years) compared with young healthy volunteers (24 to 35 years) (see Table 2). There was little difference in the Cmax for the two groups (elderly, 2.52 mcg/mL ± 0.77; young, 2.99 mcg/mL ± 1.03) (see PRECAUTIONS, Geriatric Use (≥ 65 Years of Age) ).
Pediatric Patients
Limited information is available regarding the pharmacokinetics of dosing of ketorolac tromethamine in the pediatric population. Following a single intravenous bolus dose of 0.5 mg/kg in 10 children 4 to 8 years old, the half-life was 5.8 ± 1.6 hours, the average clearance was 0.042 ± 0.01 L/hr/kg, the volume of distribution during the terminal phase (Vβ) was 0.34 ± 0.12 L/kg and the volume of distribution at steady state (Vss) was 0.26 ± 0.08 L/kg. The volume of distribution and clearance of ketorolac in pediatric patients was higher than those observed in adult subjects (see Table 1). There are no pharmacokinetic data available for administration of ketorolac tromethamine by the IM route in pediatric patients.
Renal Insufficiency
Based on single-dose data only, the mean half-life of ketorolac tromethamine in renally impaired patients is between 6 and 19 hours and is dependent on the extent of the impairment. There is poor correlation between creatinine clearance and total ketorolac tromethamine clearance in the elderly and populations with renal impairment (r = 0.5).
In patients with renal disease, the AUC∞ of each enantiomer increased by approximately 100% compared with healthy volunteers. The volume of distribution doubles for the S-enantiomer and increases by 1/5th for the R-enantiomer. The increase in volume of distribution of ketorolac tromethamine implies an increase in unbound fraction.
The AUC∞-ratio of the ketorolac tromethamine enantiomers in healthy subjects and patients remained similar, indicating there was no selective excretion of either enantiomer in patients compared to healthy subjects (see WARNINGS, Renal Effects ).
Hepatic Insufficiency
There was no significant difference in estimates of half-life, AUC∞ and Cmax in 7 patients with liver disease compared to healthy volunteers (see PRECAUTIONS, Hepatic Effect and Table 2).
Race
Pharmacokinetic differences due to race have not been identified.
| Pharmacokinetic Parameters (units) | Oral
 |
Intramuscular
 |
Intravenous Bolus
 |
|||
| 10 mg | 15 mg | 30 mg | 60 mg | 15 mg | 30 mg | |
| Bioavailability (extent) | 100% | |||||
Tmax
 |
44 ± 34 | 33 ± 21 |
44 ± 29 | 33 ± 21 |
1.1 ± 0.7 |
2.9 ± 1.8 |
Cmax
 |
0.87 ± 0.22 | 1.14 ± 0.32 |
2.42 ± 0.68 | 4.55 ± 1.27 |
2.47 ± 0.51 |
4.65 ± 0.96 |
| Cmax (mcg/mL) [steady state qid] | 1.05 ± 0.26 |
1.56 ± 0.44 |
3.11 ± 0.87 |
N/A |
3.09 ± 1.17 |
6.85 ± 2.61 |
Cmin
 |
0.29 ± 0.07 |
0.47 ± 0.13 |
0.93 ± 0.26 |
N/A | 0.61 ± 0.21 |
1.04 ± 0.35 |
Cavg
 |
0.59 ± 0.20 |
0.94 ± 0.29 |
1.88 ± 0.59 |
N/A | 1.09 ± 0.30 |
2.17 ± 0.59 |
Vβ
 |
0.175 ± 0.039 | 0.210 ± 0.044 | ||||
| % Dose metabolized ≤ 50 | % Dose excreted in feces = 6 | |||||
| % Dose excreted in urine = 91 | % Plasma protein binding = 99 | |||||
Total Clearance [in L/h/kg]
 |
Terminal Half-Life [in hours] | |||
| Type of Subjects | IM | ORAL | IM | ORAL |
| Mean (range) | Mean (range) | Mean (range) | Mean (range) | |
| Normal Subjects IM (n = 54) mean age = 32, range = 18 to 60 Oral (n = 77) mean age = 32, range = 20 to 60 |
0.023 | 0.025 | 5.3 | 5.3 |
| (0.010 to 0.046) | (0.013 to 0.050) | (3.5 to 9.2) | (2.4 to 9) | |
| Healthy Elderly Subjects IM (n = 13), Oral (n = 12) mean age = 72, range = 65 to 78 |
0.019 | 0.024 | 7 | 6.1 |
| (0.013 to 0.034) | (0.018 to 0.034) | (4.7 to 8.6) | (4.3 to 7.6) | |
| Patients With Hepatic Dysfunction IM and Oral (n = 7) mean age = 51, range = 43 to 64 |
0.029 | 0.033 | 5.4 | 4.5 |
| (0.013 to 0.066) | (0.019 to 0.051) | (2.2 to 6.9) | (1.6 to 7.6) | |
| Patients With Renal Impairment IM (n = 25), Oral (n = 9) serum creatinine = 1.9 to 5 mg/dL, mean age (IM) = 54, range = 35 to 71 mean age (Oral) = 57, range = 39 to 70 |
0.015 | 0.016 | 10.3 | 10.8 |
| (0.005 to 0.043) | (0.007 to 0.052) | (5.9 to 19.2) | (3.4 to 18.9) | |
| Renal Dialysis Patients IM and Oral (n = 9) mean age = 40, range = 27 to 63 |
0.016 | -- | 13.6 | -- |
| (0.003 to 0.036) | (8 to 39.1) | |||
IV Administration
In normal adult subjects (n = 37), the total clearance of 30 mg IV-administered ketorolac tromethamine was 0.030 (0.017 to 0.051) L/h/kg. The terminal half-life was 5.6 (4 to 7.9) hours (see Kinetics in Special Populations for use of IV dosing of ketorolac tromethamine in pediatric patients).
CLINICAL STUDIES
Adult Patients
In a postoperative study, where all patients received morphine by a PCA device, patients treated with ketorolac tromethamineIV as fixed intermittent boluses (e.g., 30 mg initial dose followed by 15 mg q3h), required significantly less morphine (26%) than the placebo group. Analgesia was significantly superior, at various postdosing pain assessment times, in the patients receiving ketorolac tromethamineIV plus PCA morphine as compared to patients receiving PCA-administered morphine alone.
Pediatric Patients
There are no data available to support the use of ketorolac tromethamine tablets in pediatric patients.
KETOROLAC TROMETHAMINE INDICATIONS AND USAGE
Carefully consider the potential benefits and risks of Ketorolac Tromethamine Tablets USP and other treatment options before deciding to use Ketorolac Tromethamine Tablets USP. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals.
Acute Pain in Adult Patients
Ketorolac Tromethamine Tablets USP are indicated for the short-term (≤ 5 days) management of moderately severe acute pain that requires analgesia at the opioid level, usually in a postoperative setting. Therapy should always be initiated with IV or IM dosing of ketorolac tromethamine and Ketorolac Tromethamine Tablets USP are to be used only as continuation treatment, if necessary.
The total combined duration of use of Ketorolac Tromethamine Tablets USP and ketorolac tromethamine is not to exceed 5 days of use because of the potential of increasing the frequency and severity of adverse reactions associated with the recommended doses (see WARNINGS , PRECAUTIONS , DOSAGE AND ADMINISTRATION , and ADVERSE REACTIONS ). Patients should be switched to alternative analgesics as soon as possible, but Ketorolac Tromethamine Tablet USP therapy is not to exceed 5 days.
KETOROLAC TROMETHAMINE CONTRAINDICATIONS
(See also Boxed WARNING.)
Ketorolac tromethamine is contraindicated in patients with previously demonstrated hypersensitivity to ketorolac tromethamine.
Ketorolac tromethamine is contraindicated in patients with active peptic ulcer disease, in patients with recent gastrointestinal bleeding or perforation and in patients with a history of peptic ulcer disease or gastrointestinal bleeding.
Ketorolac tromethamine should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients (see WARNINGS, Anaphylactoid Reactions and PRECAUTIONS, Preexisting Asthma ).
Ketorolac tromethamine is contraindicated as prophylactic analgesic before any major surgery.
Ketorolac tromethamine is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS ).
Ketorolac tromethamine is contraindicated in patients with advanced renal impairment or in patients at risk for renal failure due to volume depletion (see WARNINGS for correction of volume depletion).
Ketorolac tromethamine is contraindicated in labor and delivery because, through its prostaglandin synthesis inhibitory effect, it may adversely affect fetal circulation and inhibit uterine contractions, thus increasing the risk of uterine hemorrhage.
Ketorolac tromethamine inhibits platelet function and is, therefore, contraindicated in patients with suspected or confirmed cerebrovascular bleeding, hemorrhagic diathesis, incomplete hemostasis and those at high risk of bleeding (see WARNINGS and PRECAUTIONS ).
Ketorolac tromethamine is contraindicated in patients currently receiving aspirin or NSAIDs because of the cumulative risks of inducing serious NSAID-related adverse events.
The concomitant use of ketorolac tromethamine and probenecid is contraindicated.
The concomitant use of ketorolac tromethamine and pentoxifylline is contraindicated.
WARNINGS
(See also Boxed WARNING.)
The total combined duration of use of ketorolac tromethamine tablets and IV or IM dosing of ketorolac tromethamine is not to exceed 5 days in adults. Ketorolac tromethamine tablets are not indicated for use in pediatric patients.
The most serious risks associated with ketorolac tromethamine are:
Gastrointestinal Effects – Risk of Ulceration, Bleeding, and Perforation
Ketorolac tromethamine is contraindicated in patients with previously documented peptic ulcers and/or GI bleeding. Ketorolac tromethamine can cause serious gastrointestinal (GI) adverse events including bleeding, ulceration and perforation, of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with ketorolac tromethamine.
Only one in five patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Minor upper gastrointestinal problems, such as dyspepsia, are common and may also occur at any time during NSAID therapy. The incidence and severity of gastrointestinal complications increases with increasing dose of, and duration of treatment with, ketorolac tromethamine. Do not use ketorolac tromethamine for more than five days. However, even short-term therapy is not without risk. In addition to past history of ulcer disease, other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids, or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population.
To minimize the potential risk for an adverse GI event, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of ketorolac tromethamine until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
NSAIDs should be given with care to patients with a history of inflammatory bowel disease (ulcerative colitis, Crohn's disease) as their condition may be exacerbated.
Hemorrhage
Because prostaglandins play an important role in hemostasis and NSAIDs affect platelet aggregation as well, use of ketorolac tromethamine in patients who have coagulation disorders should be undertaken very cautiously, and those patients should be carefully monitored. Patients on therapeutic doses of anticoagulants (e.g., heparin or dicumarol derivatives) have an increased risk of bleeding complications if given ketorolac tromethamine concurrently; therefore, physicians should administer such concomitant therapy only extremely cautiously. The concurrent use of ketorolac tromethamine and therapy that affects hemostasis, including prophylactic low-dose heparin (2500 to 5000 units q12h), warfarin and dextrans have not been studied extensively, but may also be associated with an increased risk of bleeding. Until data from such studies are available, physicians should carefully weigh the benefits against the risks and use such concomitant therapy in these patients only extremely cautiously. Patients receiving therapy that affects hemostasis should be monitored closely.
In postmarketing experience, postoperative hematomas and other signs of wound bleeding have been reported in association with the peri-operative use of IV or IM dosing of ketorolac tromethamine. Therefore, peri-operative use of ketorolac tromethamine should be avoided and postoperative use be undertaken with caution when hemostasis is critical (see PRECAUTIONS ).
Renal Effects
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a NSAID may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
Ketorolac tromethamine and its metabolites are eliminated primarily by the kidneys, which, in patients with reduced creatinine clearance, will result in diminished clearance of the drug (see CLINICAL PHARMACOLOGY ). Therefore, ketorolac tromethamine should be used with caution in patients with impaired renal function (see DOSAGE AND ADMINISTRATION ) and such patients should be followed closely. With the use of ketorolac tromethamine, there have been reports of acute renal failure, interstitial nephritis and nephrotic syndrome.
Impaired Renal Function
Ketorolac tromethamine is contraindicated in patients with serum creatinine concentrations indicating advanced renal impairment (see CONTRAINDICATIONS ). Ketorolac tromethamine should be used with caution in patients with impaired renal function or a history of kidney disease because it is a potent inhibitor of prostaglandin synthesis. Because patients with underlying renal insufficiency are at increased risk of developing acute renal decompensation or failure, the risks and benefits should be assessed prior to giving ketorolac tromethamine to these patients.
Anaphylactoid Reactions
As with other NSAIDs, anaphylactoid reactions may occur in patients without a known previous exposure or hypersensitivity to ketorolac tromethamine. Ketorolac tromethamine should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs (see CONTRAINDICATIONS and PRECAUTIONS, Preexisting Asthma ). Anaphylactoid reactions, like anaphylaxis, may have a fatal outcome. Emergency help should be sought in cases where an anaphylactoid reaction occurs.
Cardiovascular Effects
Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID does increase the risk of serious GI events (see Gastrointestinal Effects – Risk of Ulceration, Bleeding, and Perforation ). Two large, controlled clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10 to 14 days following CABG surgery found an increased incidence of myocardial infarction and stroke (see CONTRAINDICATIONS ).
Hypertension
NSAIDs, including ketorolac tromethamine, can lead to onset of new hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including ketorolac tromethamine, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
Congestive Heart Failure and Edema
Fluid retention, edema, retention of NaCl, oliguria, elevations of serum urea nitrogen and creatinine have been reported in clinical trials with ketorolac tromethamine. Therefore, ketorolac tromethamine should be used only very cautiously in patients with cardiac decompensation, hypertension or similar conditions.
Skin Reactions
NSAIDs, including ketorolac tromethamine, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash, mucosal lesions, or any other sign of hypersensitivity.
Pregnancy
In late pregnancy, as with other NSAIDs, ketorolac tromethamine should be avoided because it may cause premature closure of the ductus arteriosus.
PRECAUTIONS
General
Ketorolac tromethamine cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
The pharmacological activity of ketorolac tromethamine in reducing inflammation may diminish the utility of this diagnostic sign in detecting complications of presumed noninfectious, painful conditions.
Hepatic Effect
Ketorolac tromethamine should be used with caution in patients with impaired hepatic function or a history of liver disease. Borderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs including ketorolac tromethamine. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported.
A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with ketorolac tromethamine. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), ketorolac tromethamine should be discontinued.
Hematologic Effect
Anemia is sometimes seen in patients receiving NSAIDs, including ketorolac tromethamine. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including ketorolac tromethamine, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia. NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving ketorolac tromethamine who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.
Preexisting Asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. Since cross reactivity, including bronchospasm, between aspirin and other non-steroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, ketorolac tromethamine should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.
Information for Patients
Ketorolac tromethamine is a potent NSAID and may cause serious side effects such as gastrointestinal bleeding or kidney failure, which may result in hospitalization and even fatal outcome.
Physicians, when prescribing ketorolac tromethamine, should inform their patients or their guardians of the potential risks of ketorolac tromethamine treatment (see Boxed WARNING, WARNINGS , PRECAUTIONS , and ADVERSE REACTIONS sections), instruct patients to seek medical advice if they develop treatment-related adverse events, and advise patients not to give ketorolac tromethamine tablets to other family members and to discard any unused drug.
Remember that the total combined duration of use of ketorolac tromethamine tablets and IV or IM dosing of ketorolac tromethamine is not to exceed 5 days in adults. Ketorolac tromethamine tablets are not indicated for use in pediatric patients.
Patients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
- Ketorolac tromethamine, like other NSAIDs, may cause serious CV side effects, such as MI or stroke, which may result in hospitalization and even death. Although serious CV events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative sign or symptoms. Patients should be apprised of the importance of this follow-up (see WARNINGS, Cardiovascular Effects ).
- Ketorolac tromethamine, like other NSAIDs, can cause GI discomfort and rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative sign or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up (see WARNINGS, Gastrointestinal Effects – Risk of Ulceration, Bleeding, and Perforation ).
- Ketorolac tromethamine, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS, and TEN, which may result in hospitalizations and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and should ask for medical advice when observing any indicative signs or symptoms. Patients should be advised to stop the drug immediately if they develop any type of rash and contact their physicians as soon as possible.
- Patients should promptly report signs or symptoms of unexplained weight gain or edema to their physicians.
- Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If these occur, patients should be instructed to stop therapy and seek immediate medical therapy.
- Patients should be informed of the signs of an anaphylactoid reaction (e.g., difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help (see WARNINGS ).
- In late pregnancy, as with other NSAIDs, ketorolac tromethamine should be avoided because it will cause premature closure of the ductus arteriosus.
Laboratory Tests
Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. Patients on long-term treatment with NSAIDs, should have their CBC and a chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash, etc.) or if abnormal liver tests persist or worsen, ketorolac tromethamine should be discontinued.
Drug Interactions
Ketorolac is highly bound to human plasma protein (mean 99.2%). There is no evidence in animal or human studies that ketorolac tromethamine induces or inhibits hepatic enzymes capable of metabolizing itself or other drugs.
Warfarin, Digoxin, Salicylate, and Heparin
The in vitro binding of warfarin to plasma proteins is only slightly reduced by ketorolac tromethamine (99.5% control vs 99.3%) when ketorolac plasma concentrations reach 5 to 10 mcg/mL. Ketorolac does not alter digoxin protein binding. In vitro studies indicate that, at therapeutic concentrations of salicylate (300 mcg/mL), the binding of ketorolac was reduced from approximately 99.2% to 97.5%, representing a potential twofold increase in unbound ketorolac plasma levels. Therapeutic concentrations of digoxin , warfarin , ibuprofen , naproxen , piroxicam , acetaminophen , phenytoin and tolbutamide did not alter ketorolac tromethamine protein binding.
In a study involving 12 adult volunteers, ketorolac tromethamine tablets were coadministered with a single dose of 25 mg warfarin , causing no significant changes in pharmacokinetics or pharmacodynamics of warfarin. In another study, ketorolac tromethamine dosed IV or IM was given with two doses of 5000 U of heparin to 11 healthy volunteers, resulting in a mean template bleeding time of 6.4 minutes (3.2 to 11.4 min) compared to a mean of 6 minutes (3.4 to 7.5 min) for heparin alone and 5.1 minutes (3.5 to 8.5 min) for placebo. Although these results do not indicate a significant interaction between ketorolac tromethamine and warfarin or heparin, the administration of ketorolac tromethamine to patients taking anticoagulants should be done extremely cautiously, and patients should be closely monitored (see WARNINGS and PRECAUTIONS, Hematologic Effect ).
The effects of warfarin and NSAIDs, in general, on GI bleeding are synergistic, such that the users of both drugs together have a risk of serious GI bleeding higher than the users of either drug alone.
Aspirin
When ketorolac tromethamine is administered with aspirin, its protein binding is reduced, although the clearance of free ketorolac tromethamine is not altered. The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of ketorolac tromethamine and aspirin is not generally recommended because of the potential of increased adverse effects.
Diuretics
Clinical studies, as well as postmarketing observations, have shown that ketorolac tromethamine can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure (see WARNINGS, Renal Effects ), as well as to assure diuretic efficacy.
Probenecid
Concomitant administration of ketorolac tromethamine tablets and probenecid resulted in decreased clearance and volume of distribution of ketorolac and significant increases in ketorolac plasma levels (total AUC increased approximately threefold from 5.4 to 17.8 mcg/h/mL) and terminal half-life increased approximately twofold from 6.6 to 15.1 hours. Therefore, concomitant use of ketorolac tromethamine and probenecid is contraindicated.
Lithium
NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity.
Methotrexate
NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
ACE Inhibitors/Angiotensin II Receptor Antagonists
Concomitant use of ACE inhibitors and/or angiotensin II receptor antagonists may increase the risk of renal impairment, particularly in volume-depleted patients.
Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE inhibitors and/or angiotensin II receptor antagonists. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE inhibitors and/or angiotensin II receptor antagonists.
Antiepileptic Drugs
Sporadic cases of seizures have been reported during concomitant use of ketorolac tromethamine and antiepileptic drugs (phenytoin, carbamazepine).
Psychoactive Drugs
Hallucinations have been reported when ketorolac tromethamine was used in patients taking psychoactive drugs (fluoxetine, thiothixene, alprazolam).
Pentoxifylline
When ketorolac tromethamine is administered concurrently with pentoxifylline, there is an increased tendency to bleeding.
Nondepolarizing Muscle Relaxants
In postmarketing experience there have been reports of a possible interaction between ketorolac tromethamineIV/IM and nondepolarizing muscle relaxants that resulted in apnea. The concurrent use of ketorolac tromethamine with muscle relaxants has not been formally studied.
Selective Serotonin Reuptake Inhibitors (SSRIs)
There is an increased risk of gastrointestinal bleeding when selective serotonin reuptake inhibitors (SSRIs) are combined with NSAIDs. Caution should be used when NSAIDs are administered concomitantly with SSRIs.
Carcinogenesis, Mutagenesis, Impairment of Fertility
An 18 month study in mice with oral doses of ketorolac tromethamine at 2 mg/kg/day (0.9 times the human systemic exposure at the recommended IM or IV dose of 30 mg qid, based on area-under-the-plasma-concentration curve [AUC]), and a 24 month study in rats at 5 mg/kg/day (0.5 times the human AUC) showed no evidence of tumorigenicity.
Ketorolac tromethamine was not mutagenic in the Ames test, unscheduled DNA synthesis and repair, and in forward mutation assays. Ketorolac tromethamine did not cause chromosome breakage in the in vivo mouse micronucleus assay. At 1590 mcg/mL and at higher concentrations, ketorolac tromethamine increased the incidence of chromosomal aberrations in Chinese hamster ovarian cells.
Impairment of fertility did not occur in male or female rats at oral doses of 9 mg/kg (0.9 times the human AUC) and 16 mg/kg (1.6 times the human AUC) of ketorolac tromethamine, respectively.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Reproduction studies have been performed during organogenesis using daily oral doses of ketorolac tromethamine at 3.6 mg/kg (0.37 times the human AUC) in rabbits and at 10 mg/kg (1 times the human AUC) in rats. Results of these studies did not reveal evidence of teratogenicity to the fetus. However, animal reproduction studies are not always predictive of human response.
Nonteratogenic Effects
Because of the known effects of non-steroidal anti-inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly late pregnancy) should be avoided. Oral doses of ketorolac tromethamine at 1.5 mg/kg (0.14 times the human AUC), administered after gestation Day 17, caused dystocia and higher pup mortality in rats.
There are no adequate and well-controlled studies of ketorolac tromethamine in pregnant women. Ketorolac tromethamine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery
The use of ketorolac tromethamine is contraindicated in labor and delivery because, through its prostaglandin synthesis inhibitory effect, it may adversely affect fetal circulation and inhibit uterine contractions, thus increasing the risk of uterine hemorrhage (see CONTRAINDICATIONS ).
Effects on Fertility
The use of ketorolac tromethamine, as with any drug known to inhibit cyclooxygenase/prostaglandin synthesis, may impair fertility and is not recommended in women attempting to conceive. In women who have difficulty conceiving or are undergoing investigation of infertility, withdrawal of ketorolac tromethamine should be considered.
Nursing Mothers
Limited data from one published study involving 10 breastfeeding women 2 to 6 days postpartum showed low levels of ketorolac in breast milk. Levels were undetectable (less than 5 ng/mL) in 4 of the patients. After a single administration of 10 mg of ketorolac tromethamine tablets, the maximum milk concentration observed was 7.3 ng/mL, and the maximum milk-to-plasma ratio was 0.037. After 1 day of dosing (10 mg every 6 hours), the maximum milk concentration was 7.9 ng/mL, and the maximum milk-to-plasma ratio was 0.025. Assuming a daily intake of 400 to 1,000 mL of human milk per day and a maternal body weight of 60 kg, the calculated maximum daily infant exposure was 0.00263 mg/kg/day, which is 0.4% of the maternal weight-adjusted dose.
Exercise caution when ketorolac is administered to a nursing woman. Available information has not shown any specific adverse events in nursing infants; however, instruct patients to contact their infant's health care provider if they note any adverse events.
Pediatric Use
Ketorolac tromethamine tablets are not indicated for use in pediatric patients. The safety and effectiveness of ketorolac tromethamine tablets in pediatric patients below the age of 17 have not been established.
Geriatric Use (≥ 65 Years of Age)
Because ketorolac tromethamine may be cleared more slowly by the elderly (see CLINICAL PHARMACOLOGY ) who are also more sensitive to the dose-related adverse effects of NSAIDs (see WARNINGS, Gastrointestinal Effects – Risk of Ulceration, Bleeding, and Perforation ), extreme caution, reduced dosages (see DOSAGE AND ADMINISTRATION ), and careful clinical monitoring must be used when treating the elderly with ketorolac tromethamine.
KETOROLAC TROMETHAMINE ADVERSE REACTIONS
Adverse reaction rates increase with higher doses of ketorolac tromethamine. Practitioners should be alert for the severe complications of treatment with ketorolac tromethamine, such as GI ulceration, bleeding and perforation, postoperative bleeding, acute renal failure, anaphylactic and anaphylactoid reactions and liver failure (see Boxed WARNING, WARNINGS , PRECAUTIONS , and DOSAGE AND ADMINISTRATION ). These NSAID-related complications can be serious in certain patients for whom ketorolac tromethamine is indicated, especially when the drug is used inappropriately.
In patients taking ketorolac tromethamine or other NSAIDs in clinical trials, the most frequently reported adverse experiences in approximately 1% to 10% of patients are:
| Gastrointestinal (GI) experiences including: | ||
| abdominal pain* | constipation/diarrhea | dyspepsia* |
| flatulence | GI fullness | GI ulcers (gastric/duodenal) |
| gross bleeding/perforation | heartburn | nausea* |
| stomatitis | vomiting | |
| Other experiences: | ||
| abnormal renal function | anemia | dizziness |
| drowsiness | edema | elevated liver enzymes |
| headaches* | hypertension | increased bleeding time |
| injection site pain | pruritus | purpura |
| rashes | tinnitus | sweating |
| * Incidence greater than 10% | ||
Additional adverse experiences reported occasionally (< 1% in patients taking ketorolac tromethamine or other NSAIDs in clinical trials) include:
Body as a Whole: fever, infections, sepsis
Cardiovascular: congestive heart failure, palpitation, pallor, tachycardia, syncope
Dermatologic: alopecia, photosensitivity, urticaria
Gastrointestinal: anorexia, dry mouth, eructation, esophagitis, excessive thirst, gastritis, glossitis, hematemesis, hepatitis, increased appetite, jaundice, melena, rectal bleeding
Hemic and Lymphatic: ecchymosis, eosinophilia, epistaxis, leukopenia, thrombocytopenia
Metabolic and Nutritional: weight change
Nervous System: abnormal dreams, abnormal thinking, anxiety, asthenia, confusion, depression, euphoria, extrapyramidal symptoms, hallucinations, hyperkinesis, inability to concentrate, insomnia, nervousness, paresthesia, somnolence, stupor, tremors, vertigo, malaise
Reproductive, female: infertility
Respiratory: asthma, cough, dyspnea, pulmonary edema, rhinitis
Special Senses: abnormal taste, abnormal vision, blurred vision, hearing loss
Urogenital: cystitis, dysuria, hematuria, increased urinary frequency, interstitial nephritis, oliguria/polyuria, proteinuria, renal failure, urinary retention
Other rarely observed reactions (reported from postmarketing experience in patients taking ketorolac tromethamine or other NSAIDs) are:
Body as a Whole: angioedema, death, hypersensitivity reactions such as anaphylaxis, anaphylactoid reaction, laryngeal edema, tongue edema (see WARNINGS ), myalgia
Cardiovascular: arrhythmia, bradycardia, chest pain, flushing, hypotension, myocardial infarction, vasculitis
Dermatologic: exfoliative dermatitis, erythema multiforme, Lyell’s syndrome, bullous reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis
Gastrointestinal: acute pancreatitis, liver failure, ulcerative stomatitis, exacerbation of inflammatory bowel disease (ulcerative colitis, Crohn's disease)
Hemic and Lymphatic: agranulocytosis, aplastic anemia, hemolytic anemia, lymphadenopathy, pancytopenia, postoperative wound hemorrhage (rarely requiring blood transfusion - see Boxed WARNING, WARNINGS , and PRECAUTIONS )
Metabolic and Nutritional: hyperglycemia, hyperkalemia, hyponatremia
Nervous System: aseptic meningitis, convulsions, coma, psychosis
Respiratory: bronchospasm, respiratory depression, pneumonia
Special Senses: conjunctivitis
Urogenital: flank pain with or without hematuria and/or azotemia, hemolytic uremic syndrome
Postmarketing Surveillance Study
A large postmarketing observational, nonrandomized study, involving approximately 10,000 patients receiving ketorolac tromethamineIV/IM, demonstrated that the risk of clinically serious gastrointestinal (GI) bleeding was dose-dependent (see Tables 3A and 3B). This was particularly true in elderly patients who received an average daily dose greater than 60 mg/day of ketorolac tromethamineIV/IM (see Table 3A).
| A. Adult Patients Without History of PUB |
||||
| Age of Patients | Total Daily Dose of Ketorolac Tromethamine IV/IM | |||
| ≤ 60 mg | > 60 to 90 mg | > 90 to 120 mg | > 120 mg | |
| < 65 years of age | 0.4% | 0.4% | 0.9% | 4.6% |
| ≥ 65 years of age | 1.2% | 2.8% | 2.2% | 7.7% |
| B. Adult Patients With History of PUB |
||||
| Age of Patients | Total Daily Dose of Ketorolac Tromethamine IV/IM | |||
| ≤ 60 mg | > 60 to 90 mg | > 90 to 120 mg | > 120 mg | |
| < 65 years of age | 2.1% | 4.6% | 7.8% | 15.4% |
| ≥ 65 years of age | 4.7% | 3.7% | 2.8% | 25% |
OVERDOSAGE
Symptoms and Signs
Symptoms following acute NSAID overdoses are usually limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which are generally reversible with supportive care. Gastrointestinal bleeding can occur. Hypertension, acute renal failure, respiratory depression and coma may occur, but are rare. Anaphylactoid reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose.
Treatment
Patients should be managed by symptomatic and supportive care following a NSAIDs overdose. There are no specific antidotes. Emesis and/or activated charcoal (60 g to 100 g in adults, 1 g/kg to 2 g/kg in children) and/or osmotic cathartic may be indicated in patients seen within 4 hours of ingestion with symptoms or following a large oral overdose (5 to 10 times the usual dose). Forced diuresis, alkalization of urine, hemodialysis or hemoperfusion may not be useful due to high protein binding.
Single overdoses of ketorolac tromethamine have been variously associated with abdominal pain, nausea, vomiting, hyperventilation, peptic ulcers and/or erosive gastritis and renal dysfunction which have resolved after discontinuation of dosing.
KETOROLAC TROMETHAMINE DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of ketorolac tromethamine tablets and other treatment options before deciding to use ketorolac tromethamine tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals. In adults, the combined duration of use of IV or IM dosing of ketorolac tromethamine and ketorolac tromethamine tablets is not to exceed 5 days. In adults, the use of ketorolac tromethamine tablets is only indicated as continuation therapy to IV or IM dosing of ketorolac tromethamine.
Transition from IV or IM dosing of ketorolac tromethamine (single- or multiple-dose) to multiple-dose ketorolac tromethamine tablets:
Patients age 17 to 64: 20 mg PO once followed by 10 mg q4 to 6 hours prn not > 40 mg/day
Patients age ≥ 65, renally impaired, and/or weight < 50 kg (110 lbs): 10 mg PO once followed by 10 mg q4 to 6 hours prn not > 40 mg/day
Note:
Oral formulation should not be given as an initial dose.
Use minimum effective dose for the individual patient.
Do not shorten dosing interval of 4 to 6 hours.
Total duration of treatment in adult patients: the combined duration of use of IV or IM dosing of ketorolac tromethamine and ketorolac tromethamine tablets is not to exceed 5 days.
The following table summarizes ketorolac tromethamine tablet dosing instructions in terms of age group:
| Patient Population | Ketorolac Tromethamine Tablets (following IV or IM dosing of ketorolac tromethamine) |
| Age < 17 years | Oral not approved |
| Adult Age 17 to 64 years | 20 mg once, then 10 mg q4 to 6 hours prn not > 40 mg/day |
| Adult Age ≥ 65 years, renally impaired, and/or weight < 50 kg | 10 mg once, then 10 mg q4 to 6 hours prn not > 40 mg/day |
HOW SUPPLIED
Ketorolac Tromethamine Tablets USP are available as follows:
10 mg: White, round, convex, unscored, film coated tablets, debossed "93" on one side and "314" on the other side. They are available in bottles of 10, 15, 20 and 30 tablets.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
PROTECT FROM LIGHT AND EXCESSIVE HUMIDITY.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Repackaged By :
Aidarex Pharmaceuticals LLC,
Corona, CA 92880
Rev. J 4/2013
MEDICATION GUIDE FOR NON-STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS)
Rx only
(See the end of this Medication Guide for a list of prescription NSAID medicines.)
What is the most important information I should know about medicines called Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines may increase the chance of a heart attack or stroke that can lead to death. This chance increases:
- with longer use of NSAID medicines
- in people who have heart disease
NSAID medicines should never be used right before or after a heart surgery called a "coronary artery bypass graft (CABG)."
NSAID medicines can cause ulcers and bleeding in the stomach and intestines at any time during treatment. Ulcers and bleeding:
- can happen without warning symptoms
- may cause death
The chance of a person getting an ulcer or bleeding increases with:
- taking medicines called “corticosteroids” and “anticoagulants”
- longer use
- smoking
- drinking alcohol
- older age
- having poor health
NSAID medicines should only be used:
- exactly as prescribed
- at the lowest dose possible for your treatment
- for the shortest time needed
What are Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as:
- different types of arthritis
- menstrual cramps and other types of short-term pain
Who should not take a Non-Steroidal Anti-Inflammatory Drug (NSAID)?
Do not take an NSAID medicine:
- if you had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAID medicine
- for pain right before or after heart bypass surgery
Tell your healthcare provider:
- about all of your medical conditions.
- about all of the medicines you take. NSAIDs and some other medicines can interact with each other and cause serious side effects. Keep a list of your medicines to show to your healthcare provider and pharmacist.
- if you are pregnant. NSAID medicines should not be used by pregnant women late in their pregnancy.
- if you are breastfeeding. Talk to your doctor.
What are the possible side effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
| Serious Side effects include: | Other side effects include: |
| • heart attack | • stomach pain |
| • stroke | • constipation |
| • high blood pressure | • diarrhea |
| • heart failure from body swelling (fluid retention) | • gas |
| • heartburn | |
| • kidney problems including kidney failure | • nausea |
| • bleeding and ulcers in the stomach and intestine | • vomiting |
| • low red blood cells (anemia) | • dizziness |
| • life-threatening skin reactions | |
| • life-threatening allergic reactions | |
| • liver problems including liver failure | |
| • asthma attacks in people who have asthma |
Get emergency help right away if you have any of the following symptoms:
- shortness of breath or trouble breathing
- chest pain
- weakness in one part or side of your body
- slurred speech
- swelling of the face or throat
Stop your NSAID medicine and call your healthcare provider right away if you have any of the following symptoms:
- nausea
- more tired or weaker than usual
- itching
- your skin or eyes look yellow
- stomach pain
- flu-like symptoms
- vomit blood
- there is blood in your bowel movement or it is black and sticky like tar
- unusual weight gain
- skin rash or blisters with fever
- swelling of the arms and legs, hands and feet
These are not all the side effects with NSAID medicines. Talk to your healthcare provider or pharmacist for more information about NSAID medicines. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Other information about Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
- Aspirin is an NSAID medicine but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
- Some of these NSAID medicines are sold in lower doses without a prescription (over-the-counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days.
| Generic Name | Tradename |
| Celecoxib | Celebrex |
| Diclofenac | Cataflam, Voltaren, Arthrotec (combined with misoprostol) |
| Diflunisal | Dolobid |
| Etodolac | Lodine, Lodine XL |
| Fenoprofen | Nalfon, Nalfon 200 |
| Flurbiprofen | Ansaid |
| Ibuprofen | Motrin, Tab-Profen, Vicoprofen |
| Indomethacin | Indocin, Indocin SR, Indo-Lemmon, Indomethagan |
| Ketoprofen | Oruvail |
| Ketorolac | Toradol |
| Mefenamic Acid | Ponstel |
| Meloxicam | Mobic |
| Nabumetone | Relafen |
| Naproxen | Naprosyn, Anaprox, Anaprox DS, EC-Naprosyn, Naprelan, Naprapac (copackaged with lansoprazole) |
| Oxaprozin | Daypro |
| Piroxicam | Feldene |
| Sulindac | Clinoril |
| Tolmetin | Tolectin, Tolectin DS, Tolectin 600 |
This Medication Guide has been approved by the U.S. Food and Drug Administration.
All brand names listed are the registered trademarks of their respective owners and are not trademarks of Teva Pharmaceuticals USA.
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Repackaged By :
Aidarex Pharmaceuticals LLC,
Corona, CA 92880
Rev. C 4/2013
PRINCIPAL DISPLAY PANEL

Ketorolac Tromethamine Tablets USP 10mg 30s Label Text
NDC 33261-0065-30
KETOROLAC
TROMETHAMINE
Tablets USP
10 mg
PHARMACIST: Dispense the Medication
Guide provided separately to each patient.
Rx only
30 TABLETS
Ketorolac TromethamineKetorolac Tromethamine TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||