Ketorolac Tromethamine
Ketorolac Tromethamine Ophthalmic Solution, 0.4%
FULL PRESCRIBING INFORMATION: CONTENTS*
- KETOROLAC TROMETHAMINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- KETOROLAC TROMETHAMINE INDICATIONS AND USAGE
- KETOROLAC TROMETHAMINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
STERILE OPHTHALMIC SOLUTION
KETOROLAC TROMETHAMINE DESCRIPTION
Ketorolac tromethamine is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for ophthalmic use. Its chemical name is (±)-5-benzoyl-2, 3-dihydro-1H-pyrrolizine-1-carboxylic acid compound with 2-amino-2-(hydroxytmethyl)-1,3-propanediol (1:1) and it has the following structure: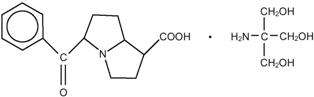
Ketorolac Tromethamine Opthalmic Solution is supplied as a sterile isotonic aqueous 0.4% solution, with a pH of 7.3 to 7.5. Ketorolac Tromethamine Ophthalmic Solution is a racemic mixture of R-(+) and S-(-)- ketorolac tromethamine. Ketorolac tromethamine may exist in three crystal forms. All forms are equally soluble in water. The pKa of ketorolac is 3.5. This white to off-white crystalline substance discolors on prolonged exposure to light. The molecular weight of ketorolac tromethamine is 376.41. The osmolality of Ketorolac Tromethamine Ophthalmic Solution is 290 mOsmol/kg.
Each mL of Ketorolac Tromethamine Ophthalmic Solution contains: Active: Ketorolac tromethamine 0.4%. Preservative: Benzalkonium chloride 0.006%. Inactives: Edetate disodium dihydrate 0.015%; octoxynol 40; sodium chloride; hydrochloric acid and/or sodium hydroxide to adjust the pH; and water for injection.
CLINICAL PHARMACOLOGY
Ketarolac tromethamine is a nonsteroidal anti-inflammatory drug which, when administered systemically, has demonstrated analgesic, anti-inflammatory, and anti-pyretic activity. The mechanism of its action is thought to be due to its ability to inhibit prostaglandin biosynthesis. Ketorolac tromethamine given systemically does not cause pupil constriction.
One drop (0.05 mL) of 0.5% ketorolac tromethamine ophthalmic solution was instilled into one eye and one drop of vehicle into the other eye TID in 26 normal subjects. Only 5 of 26 subjects had a detectable amount of ketorolac in their plasma (range 10.7 to 22.5 ng/mL) at Day 10 during topical ocular treatment. When ketorolac tromethamine 10 mg is administered systemically every 6 hours, peak plasma levels at steady state are around 960 ng/mL.
In two double-masked, multi-centered, parallel-group studies, 313 patients who had undergone photorefractive keratectomy received ketorolac tromethamine ophthalmic solution 0.4% or its vehicle QID for up to 4 days. Significant differences favored ketorolac tromethamine ophthalmic solution for the reduction of ocular pain and burning/stinging following photorefractive keratectomy surgery.
Results from clinical studies indicate that ketorolac tromethamine has no significant effect upon intraocular pressure.
The safety and effectivess of ketorolac tromethamine ophthalmic solution in post-cataract surgery patients has not been established.
KETOROLAC TROMETHAMINE INDICATIONS AND USAGE
Ketorolac tromethamine ophthalmic solution is indicated for the reduction of ocular pain and burning/stinging following corneal refraction surgery.
KETOROLAC TROMETHAMINE CONTRAINDICATIONS
Ketorolac tromethamine ophthalmic solution is contraindicated in patients with previously demonstrated hypersensitivity to any of the ingredients in the formulation.
WARNINGS
There is the potential for cross-sensitivity to acetylsalicylic acid, phenylacetic acid derivatives, and other nonsteroidal anti-inflammatory agents. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
With some nonsteroidal anti-inflammatory drugs, there exists the potential for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that ocularly applied nonsteroidal anti-inflammatory drugs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
PRECAUTIONS
All topical nonsteroidal anti-inflammatory drugs (NSAIDs) may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
Use of topical NSAIDs may result in keratitis. In some susceptible patients, continued use of topical NSAIDs may result in epithelial breakdown, corneal thinning, corneal erosion, corneal ulceration or corneal perforation. These events may be sight threatening. Patients with evidence of corneal epithelial breakdown should immediately discontinue use of topical NSAIDs and should be closely monitored for corneal health.
Postmarketing experience with topical NSAIDs suggests that patients with complicated ocular surgeries, corneal denervation, corneal epithelial defects, diabetes mellitus, ocular surface diseases (e.g., dry eye syndrome), rheumatoid arthritis, or repeat ocular surgeries within a short period of time may be at increased risk for corneal adverse events which may become sight threatening. Topical NSAIDs should be used with caution in these patients.
Postmarketing experience with topical NSAIDs also suggests that use more than 24 hours prior to surgery or use beyond 14 days post surgery may increase patient risk for the occurrence and severity of corneal adverse events.
It is recommended that ketorolac tromethamine ophthalmic solution be used with caution in patients with known bleeding tendencies or who are receiving other medications which may prolong bleeding time.
Ketorolac tromethamine ophthalmic solution should not be administered while wearing contact lenses.
Ketorolac tromethamine was not carcinogenic in rats given up to 5 mg/kg/day orally for 24 months (151 times the maximum recommended human topical ophthalmic dose, on a mg/kg basis, assuming 100% absorption in humans and animals) nor in mice given 2 mg/kg/day orally for 18 months (62.5 times the maximum recommended human topical ophthalmic dose, on a mg/kg basis, assuming 100% absorption in humans and animals).
Ketorolac tromethamine was not mutagenic in vitro in the Ames assay or in forward mutation assays. Similarly, it did not result in an in vitro increase in unscheduled DNA synthesis or an in vivo increase in chromosome breakage in mice. However, ketorolac tromethamine did result in an increased incidence in chromosomal aberrations in Chinese hamster ovary cells.
Ketorolac tromethamine did not impair fertility when administered orally to male and female rats at doses up to 280 and 499 times the maximum recommended human topical ophthalmic dose, respectively, on a mg/kg basis, assuming 100% absorption in humans and animals.
Teratogenic Effects: Pregnancy Category C. Ketorolac tromethamine, administered during organogenesis, was not teratogenic in rabbits or rats at oral doses up to 112 times and 312 times the maximum recommended human topical ophthalmic dose, respectively, on a mg/kg basis assuming 100% absorption in humans and animals. When administered to rats after Day 17 of gestation at oral doses up to 46 times the maximum recommended human topical ophthalmic dose, respectively, on a mg/kg basis, assuming 100% absorption in humans and animals, ketorolac tromethamine resulted in dystocia and increased pup mortality. There are no adequate and well-controlled studies in pregnant women. Ketorolac tromethamine ophthalmic solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects: Because of the known effects of prostaglandin-inhibiting drugs on the fetal cardiovascular system (closure of the ductus arteriosus), the use of ketorolac tromethamine ophthalmic solution during late pregnancy should be avoided.
Caution should be exercised when ketorolac tromethamine ophthalmic solution is administered to a nursing woman.
Safety and efficacy in pediatric patients below the age of 3 have not been established.
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
The most frequently reported adverse reactions for ketorolac tromethamine ophthalmic solution occurring in approximately 1 to 5% of he overall study population were conjunctival hyperemia, corneal infiltrates, headache, ocular edema and ocular pain.
The most frequent adverse events reported with the use of ketorolac tromethamine ophthalmic solutions have been transient stinging and burning on instillation. The events were reported by 20% - 40% of patients participating in these other clinical trials.
Other adverse events occurring approximately 1 to 10% of the time during treatment with ketorolac tromethamine ophthalmic solutions included allergic reactions, corneal edema, iritis, ocular inflammation, ocular irritation, superficial ocular infections, and superficial keratitis.
Clinical Practice: The following events have been identified during postmarketing use of ketorolac tromethamine ophthalmic solutions in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The events, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to topical ketorolac tromethamine ophthalmic solutions, or a combination of these factors, include corneal erosion, corneal perforation, corneal thinning and epithelial breakdown (see PRECAUTIONS , General).
The recommended dose of ketorolac tromethamine ophthalmic solution is one drop four times a day in the operated eye as needed for pain and burning/stinging, for up to 4 days following corneal refractive surgery.
Ketorolac tromethamine ophthalmic solution has been safely administered in conjunction with other ophthalmic medications such as antibiotics, beta blockers, carbonic anhydrase inhibitors, cycloplegics, and mydriatics.
Ketorolac Tromethamine Ophthalmic Solution 0.4% is supplied sterile, ina white opaque LDPE plastic bottle with a white opaque dropper and a grey opaque plastic cap as follows:
5 mL in 11 mL bottle - NDC 21695-985-05
Store at 20° - 25°C (68° - 77°F) [See USP Controlled Room Temperature]. Protect from light .
| Manufactured by: | Manufactured for: |
| Apotex Inc. | Apotex Corp. |
| Toronto, Ontario | Weston, FL 33326 |
| Canada M9L1T9 |
Repackaged:
Rebel Distributors Corp
Thousand Oaks, CA 91320
PRINCIPAL DISPLAY PANEL
Ketorolac Tromethamineketorolac tromethamine SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||