Lamivudine
HIGHLIGHTS OF PRESCRIBING INFORMATION BOXED WARNING W A RN ING: RISK OF LACTIC ACIDOSIS, EXACERBATIONS OF HEPATITIS B UPON DISCONTINUATION OF LAMIVUDINE TABLETS (HBV) , AND RISK OF HIV-1 RESISTANCE IF LAMIVUDINE TABLETS (HBV) I S USED IN PATIENTS WITH UNRECOGNIZED OR UNTREATED HIV-1 INFECTION See full prescribing information for complete boxed warning Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues. Suspend treatment if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur. ( 5.1 ) S e v er e acute exacerbations of hepatitis B have been reported in patients who have discontinued anti-hepatitis B therapy [including Lamivudine Tablets (HBV)]. Monitor hepatic function closely in these patients and, if appropriate, initiate anti-hepatitis B treatment. ( 5.2 ) Lamivudine Tablets (HBV) contain a lower dose of the same active ingredient (lamivudine) as EPIVIR® tablets used to treat HIV-1 infection. HIV-1 resistance may emerge in chronic hepatitis B patients with unrecognized or untreated HIV-1 infection because the lamivudine dosage in Lamivudine Tablets (HBV) is subtherapeutic and monotherapy is inappropriate for the treatment of HIV-1 infection. HIV counseling and testing should be offered to all patients before beginning treatment with Lamivudine Tablets (HBV) and periodically during treatment. ( 5.3 ) INDICATIONS AND USAGE Lamivudine Tablets (HBV) are a nucleoside analogue reverse transcriptase inhibitor indicated for the treatment of chronic hepatitis B virus infection associated with evidence of hepatitis B viral replication and active liver inflammation. (1) DOSAGE AND ADMINISTRATION Adult patients: 100 mg, once daily. (2.2) Pediatric patients aged 2 to 17 years: 3 mg per kg once daily up to100 mg once daily. Prescribe oral solution for pediatric patients requiring less than 100 mg daily. (2.3) Patients with renal impairment: Doses of Lamivudine Tablets (HBV) must be adjusted in accordance with renal function. (2.4) Lamivudine Tablets (HBV) should not be used with other medications that contain lamivudine or emtricitabine. (2.5) DOSAGE FORMS AND STRENGTHS Tablets: 100 mg (3) CONTRAINDICATIONSPatients with previously demonstrated clinically significant hypersensitivity (e.g., anaphylaxis) to any of the components of the products. (4)WARNINGS AND PRECAUTIONS Lamivudine Tablets (HBV) should not be used with other medications that contain lamivudine or with medications that contain emtricitabine. (5.4) Emergence of Resistance-Associated HBV Substitutions: Monitor ALT and HBV DNA levels during lamivudine treatment to aid in treatment decisions if emergence of viral mutants or loss of therapeutic response is suspected. (2.6, 5.5) Side Effects The most common reported adverse reactions in those receiving Lamivudine Tablets (HBV) (incidence greater than or equal to 10% and reported at a rate greater than placebo) were ear, nose and throat infections, sore throat, and diarrhea. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Apotex Corp. at 1-800-706-5575 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 LAMIVUDINE INDICATIONS AND USAGE
- 2 LAMIVUDINE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 LAMIVUDINE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Lactic Acidosis and Severe Hepatomegaly With Steatosis
- 5.2 Exacerbation of Hepatitis After Discontinuation of Treatment
- 5.3 Risk of HIV-1 Resistance if Lamivudine Is Used in Patients With Unrecognized or Untreated HIV-1 Infection
- 5.4 Coadministration With Other Medications Containing Lamivudine or Emtricitabine
- 5.5 Emergence of Resistance-Associated HBV Substitutions
- 6 LAMIVUDINE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 LAMIVUDINE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED
- 17 PATIENT COUNSELING INFORMATION
- PATIENT INFORMATION
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
WARNING: RISK OF LACTIC ACIDOSIS, EXACERBATIONS OF HEPATITIS B UPON DISCONTINUATION OF LAMIVUDINE TABLETS (HBV), AND RISK OF HIV-1 RESISTANCE IF LAMIVUDINE TABLETS (HBV) IS USED IN PATIENTS WITH UNRECOGNIZED OR UNTREATED HIV-1
Lactic Acidosis and Severe Hepatomegaly
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including Lamivudine Tablets (HBV). Suspend treatment if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur [ see Warnings and Precautions (5.1) ].
Exacebations of Hepatitis B Upon Discontinuation of Lamivudine Tablets (HBV)
Severe acute exacerbations of hepatitis B have been reported in patients who have discontinued anti-hepatitis B therapy [including Lamivudine Tablets (HBV)]. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue anti-hepatitis B therapy. If appropriate, initiation of anti-hepatitis B therapy may be warranted [ see Warnings and Precautions (5.2) ].
Risk of HIV-1 Resistance if Lamivudine Tablets (HBV) Is Used in Patients With Unrecognized or Untreated HIV-1 Infection
Lamivudine Tablets (HBV) is not approved for the treatment of HIV-1 infection because the lamivudine dosage in Lamivudine Tablets (HBV) is subtherapeutic and monotherapy is inappropriate for the treatment of HIV-1 infection. HIV-1 resistance may emerge in chronic hepatitis B-infected patients with unrecognized or untreated HIV-1 infection. Counseling and testing should be offered to all patients before beginning treatment with Lamivudine Tablets (HBV) and periodically during treatment [ see Warnings and Precautions (5.3) ].
1 INDICATIONS AND USAGE
Lamivudine Tablets (HBV) are indicated for the treatment of chronic hepatitis B virus (HBV) infection associated with evidence of hepatitis B viral replication and active liver inflammation [see Clinical Studies (14.1, 14.2)].
The following points should be considered when initiating therapy with Lamivudine Tablets (HBV):
- Due to high rates of resistance development in treated patients, initiation of treatment with Lamivudine Tablets (HBV) should only be considered when the use of an alternative antiviral agent with a higher genetic barrier to resistance is not available or appropriate.
- Lamivudine Tablets (HBV) have not been evaluated in patients co-infected with HIV, hepatitis C virus (HCV), or hepatitis delta virus.
- Lamivudine Tablets (HBV) have not been evaluated in liver transplant recipients or in patients with chronic hepatitis B virus infection with decompensated liver disease.
- Lamivudine Tablets (HBV) have not been evaluated in pediatric patients younger than 2 years of age with chronic HBV infection.
2 DOSAGE AND ADMINISTRATION
2.1 HIV Counseling and Testing
HIV counseling and testing should be offered to all patients before beginning treatment with Lamivudine Tablets (HBV) and periodically during treatment because of the risk of emergence of resistant-HIV-1 and limitation of treatment options if Lamivudine Tablets (HBV) is prescribed to treat chronic hepatitis B infection in a patient who has unrecognized HIV-1 infection or acquires HIV-1 infection during treatment [see Warnings and Precautions (5.3)].
2.2 Dosage in Adult Patients
The recommended oral dosage of Lamivudine Tablets (HBV) is 100 mg once daily.
2.3 Dosage in Pediatric Patients
The recommended oral dosage of Lamivudine Tablets (HBV) for pediatric patients aged 2 to 17 years is 3 mg per kg once daily up to a maximum daily dosage of 100 mg. The oral solution formulation should be prescribed for patients requiring a dosage less than 100 mg or if unable to swallow tablets.
2.4 Dosage Adjustment in Adult Patients With Renal Impairment
Dosage recommendations for adult patients with reduced renal function are provided in Table 1 [see Clinical Pharmacology (12.3)].
|
Creatinine Clearance (mL/min) |
Recommended Dosage of Lamivudine (HBV) |
| ≥50 | 100 mg once daily |
| 30-49 | 100 mg first dose, then 50 mg once daily |
| 15-29 | 100 mg first dose, then 25 mg once daily |
| 5-14 | 35 mg first dose, then 15 mg once daily |
| <5 | 35 mg first dose, then 10 mg once daily |
Following correction of the dosage for renal impairment, no additional dosage modification of Lamivudine Tablets (HBV) is required after routine (4 hour) hemodialysis or peritoneal dialysis [see Clinical Pharmacology (12.3)].
There are insufficient data to recommend a specific dosage of Lamivudine Tablets (HBV) in pediatric patients with renal impairment.
2.5 Important Administration Instructions
- Lamivudine Tablets (HBV) may be administered with or without food.
- The tablets and oral solution may be used interchangeably [see Clinical Pharmacology (12.3)].
- The oral solution should be used for doses less than 100 mg.
- Lamivudine Tablets (HBV) should not be used with other medications that contain lamivudine or medications that contain emtricitabine [see Warnings and Precautions (5.4)].
2.6 Assessing Patients During Treatment
Patients should be monitored regularly during treatment by a physician experienced in the management of chronic hepatitis B. During treatment, combinations of such events such as return of persistently elevated ALT, increasing levels of HBV DNA over time after an initial decline below assay limit, progression of clinical signs or symptoms of hepatic disease, and/or worsening of hepatic necroinflammatory findings may be considered as potentially reflecting loss of therapeutic response. Such observations should be taken into consideration when determining the advisability of continuing therapy with Lamivudine Tablets (HBV).
The optimal duration of treatment, the durability of HBeAg seroconversions occurring during treatment, and the relationship between treatment response and long-term outcomes such as hepatocellular carcinoma or decompensated cirrhosis are not known.
3 DOSAGE FORMS AND STRENGTHS
- Lamivudine Tablets (HBV): 100 mg, are orange-brown, capsule shaped, biconvex film-coated tablets engraved “APO” on one side, “LMV 100” on the other side.
4 CONTRAINDICATIONS
Lamivudine Tablets (HBV) are contraindicated in patients who have experienced a previous hypersensitivity reaction (e.g., anaphylaxis) to lamivudine or to any component of the tablets.
5 WARNINGS AND PRECAUTIONS
5.1 Lactic Acidosis and Severe Hepatomegaly With Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including lamivudine and other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Most of these reports have described patients receiving nucleoside analogues for treatment of HIV infection, but there have been reports of lactic acidosis in patients receiving lamivudine for hepatitis B. Particular caution should be exercised when administering Lamivudine Tablets (HBV) to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with Lamivudine Tablets (HBV) should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.2 Exacerbation of Hepatitis After Discontinuation of Treatment
Clinical and laboratory evidence of exacerbations of hepatitis have occurred after discontinuation of Lamivudine Tablets (HBV) (these have been primarily detected by serum ALT elevations, in addition to the re-emergence of HBV DNA commonly observed after stopping treatment; see Table 4 for more information regarding frequency of posttreatment ALT elevations) [see Adverse Reactions (6.1)]. Although most events appear to have been self-limited, fatalities have been reported in some cases. The causal relationship of hepatitis exacerbation after discontinuation of lamivudine has not been clearly established. Patients should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment with Lamivudine Tablets (HBV). There is insufficient evidence to determine whether re-initiation of lamivudine alters the course of posttreatment exacerbations of hepatitis.
5.3 Risk of HIV-1 Resistance if Lamivudine Is Used in Patients With Unrecognized or Untreated HIV-1 Infection
Lamivudine Tablets (HBV) contain a lower lamivudine dose than the lamivudine dose in the following drugs used to treat HIV-1 infection:
- EPIVIR® tablets and oral solution,
- COMBIVIR® (lamivudine/zidovudine) tablets,
- EPZICOM® (abacavir sulfate and lamivudine) tablets, and
- TRIZIVIR® (abacavir, lamivudine, and zidovudine) tablets.
The formulation and dosage of lamivudine in Lamivudine Tablets (HBV) are not approved for patients co-infected with HBV and HIV. If a decision is made to administer lamivudine to such patients, the higher dosage indicated for HIV therapy should be used as part of an appropriate combination regimen, and the prescribing information for EPIVIR®, COMBIVIR®, EPZICOM®, or TRIZIVIR®, as well as for Lamivudine Tablets (HBV), should be consulted. HIV counseling and testing should be offered to all patients before beginning Lamivudine Tablets (HBV) and periodically during treatment because of the risk of rapid emergence of resistant HIV and limitation of treatment options if Lamivudine Tablets (HBV) is prescribed to treat chronic hepatitis B in a patient who has unrecognized or untreated HIV-1 infection or acquires HIV-1 infection during treatment.
5.4 Coadministration With Other Medications Containing Lamivudine or Emtricitabine
Do not coadminister Lamivudine Tablets (HBV) with other lamivudine-containing products including EPIVIR® (lamivudine), COMBIVIR® (lamivudine/zidovudine), EPZICOM® (abacavir/lamivudine), or TRIZIVIR® (abacavir/lamivudine/zidovudine).
Do not coadminister Lamivudine Tablets (HBV) with emtricitabine-containing products including ATRIPLA® (efavirenz/emtricitabine/tenofovir disoproxil fumarate), COMPLERA® (rilpivirine/emtricitabine/tenofovir disoproxil fumarate), EMTRIVA® (emtricitabine), STRIBILD® (elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate), or TRUVADA® (emtricitabine/tenofovir disoproxil fumarate).
5.5 Emergence of Resistance-Associated HBV Substitutions
In controlled clinical trials, YMDD-mutant HBV was detected in subjects with on–lamivudine re-appearance of HBV DNA after an initial decline below the solution-hybridization assay limit [see Microbiology (12.4)]. Subjects treated with lamivudine (adults and children) with YMDD-mutant HBV at 52 weeks showed diminished treatment responses in comparison with subjects treated with lamivudine without evidence of YMDD substitutions, including the following: lower rates of HBeAg seroconversion and HBeAg loss (no greater than placebo recipients), more frequent return of positive HBV DNA, and more frequent ALT elevations. In the controlled trials, when subjects developed YMDD-mutant HBV, they had a rise in HBV DNA and ALT from their own previous on-treatment levels. Progression of hepatitis B, including death, has been reported in some subjects with YMDD-mutant HBV, including subjects from the liver transplant setting and from other clinical trials. In clinical practice, monitoring of ALT and HBV DNA levels during treatment with Lamivudine Tablets (HBV) may aid in treatment decisions if emergence of viral mutants is suspected.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Lactic acidosis and severe hepatomegaly with steatosis [see Warnings and Precautions (5.1)].
- Exacerbation of hepatitis B after discontinuation of treatment [see Warnings and Precautions (5.2)].
- Risk of emergence of resistant HIV-1 infection [see Warnings and Precautions (5.3)].
- Risk of emergence of resistant HIV-1 infection [see Warnings and Precautions (5.3)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Clinical Trials of Adults With Chronic Hepatitis B Virus Infection: Clinical adverse reactions (regardless of investigator’s causality assessment) reported in greater or equal to 10% of subjects who received lamivudine and reported at a rate greater than placebo are listed in Table 2.
| a Includes adverse events regardless of severity and causality assessment. | ||
| Adverse Event |
Lamivudine (n = 332) |
Placebo (n = 200) |
| Ear, Nose, and Throat | ||
| Ear, nose, and throat infections | 25% | 21% |
| Sore throat | 13% | 8% |
| Gastrointestinal | ||
| Diarrhea | 14% | 12% |
Specified laboratory abnormalities reported in subjects who received lamivudine and reported at a rate greater than in subjects who received placebo are listed in Table 3.
| a Includes subjects treated for 52 to 68 weeks. | ||
| b Includes observations during and after treatment in the 2 placebo-controlled trials that collected this information. | ||
| ULN = Upper limit of normal. | ||
|
Test (Abnormal Level) |
Subjects With Abnormality/Patients With Observations | |
| Lamivudine | Placebo | |
| Serum Lipase ≥2.5 x ULNb | 10% | 7% |
| CPK ≥7 x baseline | 9% | 5% |
| Platelets <50,000/mm3 | 4% | 3% |
In subjects followed for up to 16 weeks after discontinuation of treatment, posttreatment ALT elevations were observed more frequently in subjects who had received lamivudine 100 mg than in subjects who had received placebo. A comparison of ALT elevations between Weeks 52 and 68 in subjects who discontinued lamivudine 100 mg at Week 52 and subjects in the same trials who received placebo throughout the treatment course is shown in Table 4.
| a Each subjec may be represented in one or more category. | ||
|
b During treatment phase. c Comparable to a Grade 3 toxicity in accordance with modified WHO criteria. |
||
| ULN = Upper limit of normal. | ||
| Abnormal Value | Subjects With ALT Elevation/ Subjects With Observationsa |
|
| Lamivudineb | Placebob | |
| ALT ≥2 x baseline value | 27% | 19% |
| ALT ≥3 x baseline valuec | 21% | 8% |
| ALT ≥2 x baseline value and absolute ALT >500 IU/L | 15% | 7% |
| ALT ≥2 x baseline value; and bilirubin >2 x ULN and ≥2 x baseline value | 0.7% | 0.9% |
Adverse Reactions in Clinical Trials of Pediatric Subjects With Chronic Hepatitis B Virus Infection: Most commonly observed adverse reactions in the pediatric trials were similar to those in adult trials. Posttreatment transaminase elevations were observed in some subjects followed after cessation of lamivudine.
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been reported during postmarketing use of lamivudine. Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure. These reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to lamivudine.
Blood and Lympatic System Disorders: Thrombocytopenia.
Digestive: Stomatitis.
Endocrine and Metabolic: Hyperglycemia.
General: Weakness.
Blood and Lymphatic: Anemia (including pure red cell aplasia and severe anemias progressing on therapy), lymphadenopathy, splenomegaly.
Hepatic and Pancreatic: Lactic acidosis and steatosis, posttreatment exacerbation of hepatitis [see Boxed Warning], pancreatitis.
Hypersensitivity: Anaphylaxis, urticaria.
Musculoskeletal: Cramps, rhabdomyolysis.
Nervous: Paresthesia, peripheral neuropathy.
Respiratory: Abnormal breath sounds/wheezing.
Skin: Alopecia, pruritus, rash.
7 DRUG INTERACTIONS
Lamivudine is predominantly eliminated in the urine by active organic cationic secretion. The possibility of interactions with other drugs administered concurrently should be considered, particularly when their main route of elimination is active renal secretion via the organic cationic transport system (e.g., trimethoprim). No data are available regarding interactions with other drugs that have renal clearance mechanisms similar to that of lamivudine.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled trials of lamivudine in pregnant women. Because animal reproduction studies are not always predictive of human response, Lamivudine Tablets (HBV) should be used during pregnancy only if the potential benefits outweigh the potential risks to the fetus.
Antiretroviral Pregnancy Registry: To monitor maternal-fetal outcomes of pregnant women exposed to lamivudine, a Pregnancy Registry has been established. Healthcare providers are encouraged to register patients by calling 1-800-258-4263.
Animal Data: Animal reproduction studies in rats and rabbits revealed no evidence of teratogenicity. Reproduction studies have been performed in rats and rabbits at orally administered doses up to 4,000 mg/kg/day and 1,000 mg/kg/day, respectively, producing plasma levels up to approximately 60 times that for the adult HBV dose. Evidence of early embryolethality was seen in the rabbit at exposure levels similar to those observed in humans, but there was no indication of this effect in the rat at exposure levels up to 60 times those in humans.
Studies in pregnant rats and rabbits showed that lamivudine is transferred to the fetus through the placenta.
8.3 Nursing Mothers
Lamivudine is excreted in human milk. Samples of breast milk obtained from 20 mothers receiving lamivudine monotherapy (300 mg twice daily, 6 times the recommended dosage for hepatitis B infection) or combination therapy (150 mg lamivudine twice daily [3 times the recommended dosage for hepatitis B infection] and 300 mg zidovudine twice daily) had measurable concentrations of lamivudine.
Because of the potential for serious adverse reactions in nursing infants, a decision should be made to discontinue Lamivudine Tablets (HBV) taking into consideration the importance of continued hepatitis B therapy to the mother and the known benefits of breastfeeding.
8.4 Pediatric Use
Lamivudine Tablets (HBV) is indicated for the treatment of chronic hepatitis B virus infection in pediatric patients aged 2 to 17 years [see Indications and Usage (1), Clinical Pharmacology (12.3), Clinical Studies (14.2)]. The safety and efficacy of Lamivudine Tablets (HBV) in pediatric patients younger than 2 years have not been established.
8.5 Geriatric Use
Clinical trials of lamivudine 100 mg did not include sufficient numbers of subjects aged and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. In particular, because lamivudine is substantially excreted by the kidney and elderly patients are more likely to have decreased renal function, renal function should be monitored and dosage adjustments should be made accordingly [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].
8.6 Patients With Impaired Renal Function
Reduction of the dosage of Lamivudine Tablets (HBV) is recommended for patients with impaired renal function [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].
8.7 Patients With Impaired Liver Function
No dose adjustment for lamivudine is required for patients with impaired hepatic function.
10 OVERDOSAGE
There is no known antidote for lamivudine. If overdose occurs, the patient should be monitored, and standard supportive treatment utilized, as required.
Because a negligible amount of lamivudine was removed via (4-hour) hemodialysis, continuous ambulatory peritoneal dialysis, and automated peritoneal dialysis, it is not known if continuous hemodialysis would provide clinical benefit in a lamivudine overdose event.
11 DESCRIPTION
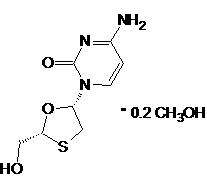
Lamivudine Tablets (HBV) is a synthetic nucleoside analogue with activity against HBV. The drug substance used in Lamivudine Tablets (HBV) is lamivudine in the form of lamivudine methanol solvate. The chemical name of lamivudine methanol solvate is (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl) )2(1H)-pyrimidin-2-one methanol solvate. Lamivudine is the (-)enantiomer of a dideoxy analogue of cytidine. Lamivudine has also been referred to as (-)2′,3′-dideoxy, 3′-thiacytidine. It has a molecular formula of C8H11N3O3S• O.2 CH4O C8H11N3O3S and a molecular weight of 229235.66.3. It has the following structural formula:
Lamivudine is a white to off-white crystalline solidpowder with a solubility of approximately 70 mg per mL in water at 20°C. It is highly soluble in water.
Lamivudine Tablets (HBV) tablets are for oral administration. Each tablet contains lamivudine methanol solvate equivalent to 100 mg of lamivudine and the inactive ingredients anhydrous lactose, crospovidone, colloidal silicon dioxide, magnesium stearate, hypromellose, hydroxypropyl cellulose, polyethylene glycol, titanium dioxide, red ferric oxide and yellow ferric oxide.100 mg of lamivudine and the inactive ingredients hypromellose, macrogol 400, magnesium stearate, microcrystalline cellulose, polysorbate 80, red iron oxide, sodium starch glycolate, titanium dioxide, and yellow iron oxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lamivudine is an antiviral agent [see Microbiology (12.4)].
12.3 Pharmacokinetics
Pharmacokinetics in Adults: The pharmacokinetic properties of lamivudine have been studied as single and multiple oral doses ranging from 5 mg to 600 mg per day administered to HBV-infected subjects.
Absorption and Bioavailability: Following single oral doses of 100 mg, the peak serum lamivudine concentration (Cmax) in HBV-infected patients (steady state) and healthy subjects (single dose) was 1.28 ± 0.56 mcg per mL and 1.05 ± 0.32 mcg per mL (mean ± SD), respectively, which occurred between 0.5 and 2 hours after administration. The area under the plasma concentration versus time curve (AUC[0-24 h]) following 100 mg lamivudine oral single and repeated daily doses to steady state was 4.3 ± 1.4 (mean ± SD) and 4.7 ± 1.7 mcg•hour per mL, respectively. The relative bioavailability of the tablet and oral solution were demonstrated in healthy subjects. Although the solution demonstrated a slightly higher peak serum concentration (Cmax), there was no significant difference in systemic exposure (AUC) between the oral solution and the tablet. Therefore, the oral solution and the tablet may be used interchangeably.
After oral administration of lamivudine once daily to HBV-infected adults, the AUC and Cmax increased in proportion to dose over the range from 5 mg to 600 mg once daily.
Absolute bioavailability in 12 adult subjects was 86% ± 16% (mean ± SD) for the 150 mg tablet and 87% ± 13% for the 10 mg per mL oral solution.
Effects of Food on Oral Absorption: The 100-mg tablet was administered orally to 24 healthy subjects on 2 occasions, once in the fasted state and once with food (standard meal: 967 kcal; 67 grams fat, 33 grams protein, 58 grams carbohydrate). There was no significant difference in systemic exposure (AUC) in the fed and fasted states.
Distribution: The apparent volume of distribution after IV administration of lamivudine to 20 asymptomatic HIV-1-infected subjects was 1.3 ± 0.4 L per kg, suggesting that lamivudine distributes into extravascular spaces. Volume of distribution was independent of dose and did not correlate with body weight.
Binding of lamivudine to human plasma proteins is less than 36% and independent of dose. In vitro studies showed that over the concentration range of 0.1 to 100 mcg per mL, the amount of lamivudine associated with erythrocytes ranged from 53% to 57% and was independent of concentration.
Metabolism: Metabolism of lamivudine is a minor route of elimination. In humans, the only known metabolite of lamivudine is the trans-sulfoxide metabolite. In 9 healthy subjects receiving 300 mg of lamivudine as single oral doses, a total of 4.2% (range: 1.5% to 7.5%) of the dose was excreted as the trans-sulfoxide metabolite in the urine, the majority of which was excreted in the first 12 hours. Serum concentrations of the trans-sulfoxide metabolite have not been determined.
Elimination: The majority of lamivudine is eliminated unchanged in urine by active organic cationic secretion. In 9 healthy subjects given a single 300 mg oral dose of lamivudine, renal clearance was 199.7 ± 56.9 mL per min (mean ± SD). In 20 HIV-1-infected subjects given a single IV dose, renal clearance was 280.4 ± 75.2 mL per min (mean ± SD), representing 71% ± 16% (mean ± SD) of total clearance of lamivudine.
In most single-dose trials in HIV-1-infected subjects, HBV-infected subjects, or healthy subjects with serum sampling for 24 hours after dosing, the observed mean elimination half-life (t½) ranged from 5 to 7 hours. In HIV-1-infected subjects, total clearance was 398.5 ± 69.1 mL per min (mean ± SD). Oral clearance and elimination half-life were independent of dose and body weight over an oral dosing range of 0.25 to 10 mg per kg.
Special Populations: Adults With Renal Impairment: The pharmacokinetic properties of lamivudine have been determined in healthy subjects and in subjects with impaired renal function, with and without hemodialysis (Table 5).
| Parameter |
Creatinine Clearance Criterion (Number of Subjects) |
||
|
≥80 mL/min (n = 9) |
20-59 mL/min (n = 8) |
<20 mL/min (n = 6) |
|
| Creatinine clearance (mL/min) |
97 (range 82-117) |
39 (range 25-49) |
15 (range 13-19) |
| Cmax (mcg/mL) | 1.31 ± 0.35 | 1.85 ± 0.40 | 1.55 ± 0.31 |
| AUC (mcg•hr/mL) | 5.28 ± 1.01 | 14.67 ± 3.74 | 27.33 ± 6.56 |
| Cl/F (mL/min) | 326.4 ± 63.8 | 120.1 ± 29.5 | 64.5 ± 18.3 |
Exposure (AUC), Cmax, and half-life increased with diminishing renal function (as expressed by creatinine clearance). Apparent total oral clearance (Cl/F) of lamivudine decreased as creatinine clearance decreased. Tmax was not significantly affected by renal function. Based on these observations, it is recommended that the dosage of lamivudine be modified in patients with renal impairment [see Dosage and Administration (2.4)].
Hemodialysis increases lamivudine clearance from a mean of 64 to 88 mL per min; however, the length of time of hemodialysis (4 hours) was insufficient to significantly alter mean lamivudine exposure after a single-dose administration. Continuous ambulatory peritoneal dialysis and automated peritoneal dialysis have negligible effects on lamivudine clearance. Therefore, it is recommended, following correction of dose for creatinine clearance, that no additional dose modification be made after routine hemodialysis or peritoneal dialysis.
It is not known whether lamivudine can be removed by continuous (24-hour) hemodialysis.
Pediatric Patients With Renal Impairment: The effect of renal impairment on lamivudine pharmacokinetics in pediatric patients with chronic hepatitis B is not known.
Adults With Hepatic Impairment: The pharmacokinetic properties of lamivudine in adults with hepatic impairment are shown in Table 6). Subjects were stratified by severity of hepatic impairment.
| a Hepatic impairment assessed by aminopyrine breath test. | |||
| Parameter |
Normal (n = 8) |
Impairmenta | |
|
Moderate (n = 8) |
Severe (n = 8) |
||
| Cmax (mcg/mL) | 0.92 ± 0.31 | 1.06 ± 0.58 | 1.08 ± 0.27 |
| AUC (mcg•hr/mL) | 3.96 ± 0.58 | 3.97 ± 1.36 | 4.30 ± 0.63 |
| Tmax (hr) | 1.3 ± 0.8 | 1.4 ± 0.8 | 1.4 ± 1.2 |
| Cl/F (mL/min) | 424.7 ± 61.9 | 456.9 ± 129.8 | 395.2 ± 51.8 |
| Clr (mL/min) | 279.2 ± 79.2 | 323.5 ± 100.9 | 216.1 ± 58.0 |
Pharmacokinetic parameters were not altered by diminishing hepatic impairment. Therefore, no dose adjustment for lamivudine is required for patients with impaired hepatic function. Safety and efficacy of lamivudine have not been established in the presence of decompensated liver disease [see Indications and Usage (1)].
Adults Post-Hepatic Transplant: Fourteen HBV-infected subjects received liver transplant following lamivudine therapy and completed pharmacokinetic assessments at enrollment, 2 weeks after 100 mg once-daily dosing (pre-transplant), and 3 months following transplant; there were no significant differences in pharmacokinetic parameters. The overall exposure of lamivudine is primarily affected by renal impairment; consequently, transplant patients with renal impairment had generally higher exposure than patients with normal renal function. Safety and efficacy of lamivudine have not been established in this population [see Indications and Usage (1)].
Pediatric Subjects: Lamivudine pharmacokinetics were evaluated in a 28 day dose-ranging trial in 53 pediatric subjects with chronic hepatitis B. Subjects aged 2 to 12 years were randomized to receive lamivudine 0.35 mg per kg twice daily, 3 mg per kg once daily, 1.5 mg per kg twice daily, or 4 mg per kg twice daily. Subjects aged 13 to 17 years received lamivudine 100 mg once daily. Lamivudine Tmax was 0.5 to 1 hour. In general, both Cmax and exposure (AUC) showed dose proportionality in the dosing range studied. Weight-corrected oral clearance was highest at age 2 and declined from 2 to 12 years, where values were then similar to those seen in adults. A dose of 3 mg per kg given once daily produced a steady-state lamivudine AUC (mean 5,953 ng•hour per mL ± 1,562 SD) similar to that associated with a dose of 100 mg per day in adults.
Gender: There are no significant gender differences in lamivudine pharmacokinetics.
Race: There are no significant racial differences in lamivudine pharmacokinetics.
Drug Interactions: Interferon Alfa: Multiple doses of lamivudine and a single dose of interferon were coadministered to 19 healthy male subjects in a pharmacokinetics trial. Results indicated a 10% reduction in lamivudine AUC, but no change in interferon pharmacokinetic parameters when the 2 drugs were given in combination. All other pharmacokinetic parameters (Cmax, Tmax, and t½) were unchanged. There was no significant pharmacokinetic interaction between lamivudine and interferon alfa in this trial.
Ribavirin: In vitro data indicate ribavirin reduces phosphorylation of lamivudine, stavudine, and zidovudine. However, no pharmacokinetic (e.g., plasma concentrations or intracellular triphosphorylated active metabolite concentrations) or pharmacodynamic (e.g., loss of HIV-1/HCV virologic suppression) interaction was observed when ribavirin and lamivudine (n = 18), stavudine (n = 10), or zidovudine (n = 6) were coadministered as part of a multi-drug regimen to HIV-1/HCV co-infected subjects.
Trimethoprim/Sulfamethoxazole: Lamivudine and trimethoprim/sulfamethoxazole (TMP/SMX) were coadministered to 14 HIV-positive subjects in a single-center, open-label, randomized, crossover trial. Each subject received treatment with a single 300 mg dose of lamivudine and TMP 160 mg/SMX 800 mg once a day for 5 days with concomitant administration of lamivudine 300 mg with the fifth dose in a crossover design. Coadministration of TMP/SMX with lamivudine resulted in an increase of 44% ± 23% (mean ± SD) in lamivudine AUC, a decrease of 29% ± 13% in lamivudine oral clearance, and a decrease of 30% ± 36% in lamivudine renal clearance. The pharmacokinetic properties of TMP and SMX were not altered by coadministration with lamivudine.
Zidovudine: Lamivudine and zidovudine were coadministered to 12 asymptomatic HIV-positive adult subjects in a single-center, open-label, randomized, crossover trial. No significant differences were observed in AUC or total clearance for lamivudine or zidovudine when the 2 drugs were administered together. Coadministration of lamivudine with zidovudine resulted in an increase of 39% ± 62% (mean ± SD) in Cmax of zidovudine.
12.4 Microbiology
Mechanism of Action: Lamivudine is a synthetic nucleoside analogue. Intracellularly, lamivudine is phosphorylated to its active 5′-triphosphate metabolite, lamivudine triphosphate, 3TC-TP. The principal mode of action of 3TC-TP is the inhibition of the RNA- and DNA dependent polymerase activities of HBV reverse transcriptase (rt) via DNA chain termination after incorporation of the nucleotide analogue into viral DNA. 3TC-TP is a weak inhibitor of mammalian α, β, and γ-DNA polymerases.
Antiviral Activity: Activity of lamivudine against HBV in cell culture was assessed in HBV DNA-transfected 2.2.15 cells, HB611 cells, and infected human primary hepatocytes. EC50 values (the concentration of drug needed to reduce the level of extracellular HBV DNA by 50%) varied from 0.01 microM (2.3 ng per mL) to 5.6 microM (1.3 mcg per mL) depending upon the duration of exposure of cells to lamivudine, the cell model system, and the protocol used. See the EPIVIR® prescribing information for information regarding activity of lamivudine against HIV.
Resistance: Lamivudine-resistant isolates were identified in subjects with virologic breakthrough, defined when using solution hybridization assay as the detection of HBV DNA in serum on 2 or more occasions after failing to detect HBV DNA on 2 or more occasions and defined when using PCR assay as a greater than 1 log10 (10-fold) increase in serum HBV DNA from nadir during treatment in a subject who had an initial virologic response.
Lamivudine-resistant HBV isolates develop rtM204V/I substitutions in the YMDD motif of the catalytic domain of the viral reverse transcriptase. rtM204V/I substitutions are frequently accompanied by other substitutions (rtV173L, rtL180M) which enhance the level of lamivudine resistance or act as compensatory substitutions improving replication efficiency. Other substitutions detected in lamivudine-resistant HBV isolates include rtL80I and rtA181T.
In 4 controlled clinical trials in adults with HBeAg-positive chronic hepatitis B virus infection (CHB), YMDD-mutant HBV was detected in 81 of 335 subjects receiving lamivudine100 mg once daily for 52 weeks. The prevalence of YMDD substitutions was less than 10% in each of these trials for subjects studied at 24 weeks and increased to an average of 24% (range in 4 trials: 16% to 32%) at 52 weeks. In limited data from a long-term follow-up trial in subjects who continued 100 mg per day lamivudine after one of these trials, YMDD substitutions further increased from 18% (10 of 57) at 1 year to 41% (20 of 49), 53% (27 of 51), and 69% (31 of 45) after 2, 3, and 4 years of treatment, respectively. Over the 5-year treatment period, the proportion of subjects who developed YMDD-mutant HBV at any time was 69% (40 of 58).
In a controlled trial, treatment-naive subjects with HBeAg-positive CHB were treated with lamivudine or lamivudine plus adefovir dipivoxil combination therapy. Following 104 weeks of therapy, YMDD-mutant HBV was detected in 7 of 40 (18%) subjects receiving combination therapy compared with 15 of 35 (43%) subjects receiving therapy with only lamivudine. In another controlled trial, combination therapy was evaluated in adult subjects with HBeAg-positive CHB who had YMDD-mutant HBV and diminished clinical and virologic response tolamivudine Following 52 weeks of lamivudine plus adefovir dipivoxil combination therapy (n = 46) or therapy with only lamivudine (n = 49), YMDD-mutant HBV was detected less frequently in subjects receiving combination therapy, 62% versus 96%.
A published trial suggested that the rates of lamivudine resistance in subjects treated for HBeAg-negative CHB appear to be more variable (0% to 27% at 1 year and 10% to 56% at 2 years).
Pediatric Subjects: In a controlled trial in pediatric subjects, YMDD-mutant HBV was detected in 31 of 166 (19%) subjects receiving lamivudine for 52 weeks. For a subgroup that remained on therapy with lamivudine in a follow-up trial, YMDD substitutions increased from 24% (29 of 121) at 12 months to 59% (68 of 115) at 24 months and 64% (66 of 103) at 36 months of treatment with lamivudine.
Cross-Resistance: HBV containing lamivudine resistance-associated substitutions (rtL180M, rtM204I, rtM204V, rtL180M and rtM204V, rtV173L and rtL180M and rtM204V) retain susceptibility to adefovir dipivoxil but have reduced susceptibility to entecavir (30 fold) and telbivudine (greater than 100 fold). The lamivudine resistance-associated substitution rtA181T results in diminished response to adefovir and telbivudine. Similarly, HBV with entecavir resistance-associated substitutions (I169T/M250V and T184G/S202I) have greater than 1,000 fold reductions in susceptibility to lamivudine.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Long-term carcinogenicity studies with lamivudine in mice and rats showed no evidence of carcinogenic potential at exposures up to 34 times (mice) and 200 times (rats) those observed in humans at the recommended therapeutic dose for chronic hepatitis B.
Mutagenesis: Lamivudine was not active in a microbial mutagenicity screen or an in vitro cell transformation assay, but showed weak in vitro mutagenic activity in a cytogenetic assay using cultured human lymphocytes and in the mouse lymphoma assay. However, lamivudine showed no evidence of in vivo genotoxic activity in the rat at oral doses of up to 2,000 mg per kg producing plasma levels of 60 to 70 times those in humans at the recommended dose for chronic hepatitis B.
Impairment of Fertility: In a study of reproductive performance, lamivudine administered to rats at doses up to 4,000 mg per kg per day, producing plasma levels 80 to 120 times those in humans, revealed no evidence of impaired fertility and no effect on the survival, growth, and development to weaning of the offspring.
14 CLINICAL STUDIES
14.1 Clinical Studies of Lamivudine in Adult Patients
The safety and efficacy of lamivudine100 mg once daily versus placebo were evaluated in 3 controlled trials in subjects with compensated chronic hepatitis B virus infection. All subjects were aged 16 years or older and had chronic hepatitis B virus infection (serum HBsAg-positive for at least 6 months) accompanied by evidence of HBV replication (serum HBeAg-positive and positive for serum HBV DNA) and persistently elevated ALT levels and/or chronic inflammation on liver biopsy compatible with a diagnosis of chronic viral hepatitis. The results of these trials are summarized below.
- Trial 1 was a randomized, double-blind trial of lamivudine 100 mg once daily versus placebo for 52 weeks followed by a 16-week no-treatment period in 141 treatment-naive US subjects.
- Trial 2 was a randomized, double-blind, 3-arm trial that compared lamivudine 25 mg once daily versus lamivudine 100 mg once daily versus placebo for 52 weeks in 358 Asian subjects.
- Trial 3 was a randomized, partially-blind trial conducted primarily in North America and Europe in 238 subjects who had ongoing evidence of active chronic hepatitis B despite previous treatment with interferon alfa. The trial compared lamivudine 100 mg once daily for 52 weeks, followed by either lamivudine 100 mg or matching placebo once daily for 16 weeks (Arm 1), versus placebo once daily for 68 weeks (Arm 2).
Principal endpoint comparisons for the histologic and serologic outcomes in subjects receiving lamivudine (100 mg daily) or placebo in these trials are shown in the following tables.
| a Improvement was defined as a greater than or equal to 2-point decrease in the Knodell Histologic Activity Index (HAI) at Week 52 compared with pretreatment HAI. Subjects with missing data at baseline were excluded. | ||||||
| Assessment | Trial 1 | Trial 2 | Trial 3 | |||
| Lamivudine 100 mg(n = 62) |
Placebo (n = 63) |
Lamivudine 100 mg(n = 131) |
Placebo (n = 68) |
Lamivudine 100 mg (n = 110) |
Placebo |
|
| Improvementa | 55% | 25% | 56% | 26% | 56% | 26% |
| No Improvement | 27% | 59% | 36% | 62% | 25% | 54% |
| Missing Data | 18% | 16% | 8% | 12% | 19% | 20% |
| a Three-component seroconversion was defined as Week 52 values showing loss of HBeAg, gain of HBeAb, and reduction of HBV DNA to below the solution-hybridization assay limit. Subjects with negative baseline HBeAg or HBV DNA assay were excluded from the analysis. | ||||||
| Seroconversion | Trial 1 | Trial 2 | Trial 3 | |||
|
Lamivudine (n = 63) |
Placebo (n = 69) |
Lamivudine (n = 140) |
Placebo (n = 70) |
Lamivudine (n = 108) |
Placebo (n = 53) |
|
| Seroconverters | 17% | 6% | 16% | 4% | 15% | 13% |
Normalization of serum ALT levels was more frequent with lamivudine treatment compared with placebo in Trials 1-3.
The majority of subjects treated with lamivudine showed a decrease of HBV DNA to below the assay limit early in the course of therapy. However, reappearance of assay-detectable HBV DNA during treatment with lamivudine was observed in approximately one-third of subjects after this initial response.
14.2 Clinical Studies of Lamivudine in Pediatric Subjects
The safety and efficacy of lamivudine were evaluated in a double-blind clinical trial in 286 subjects aged from 2 to 17 years, who were randomized (2:1) to receive 52 weeks of lamivudine (3 mg per kg once daily to a maximum of 100 mg once daily) or placebo. All subjects had compensated chronic hepatitis B accompanied by evidence of hepatitis B virus replication (positive serum HBeAg and positive for serum HBV DNA by a research branched-chain DNA assay) and persistently elevated serum ALT levels. The combination of loss of HBeAg and reduction of HBV DNA to below the assay limit of the research assay, evaluated at Week 52, was observed in 23% of subjects treated with lamivudine and 13% of placebo-treated subjects. Normalization of serum ALT was achieved and maintained to Week 52 more frequently in subjects treated with lamivudine compared with placebo (55% versus13%). As in the adult controlled trials, most subjects treated with lamivudine had decreases in HBV DNA below the assay limit early in treatment, but about one-third of subjects with this initial response had reappearance of assay-detectable HBV DNA during treatment. Adolescents(aged 13 to 17 years) showed less evidence of treatment effect than younger pediatric subjects.
16 HOW SUPPLIED
Lamivudine Tablets (HBV), 100 mg, are orange-brown, capsule shaped, biconvex film-coated tablets engraved “APO” on one side, “LMV 100” on the other side.
Box of 30 Unit Dose tablets (NDC 0179-0152-70.
Store at 20º to 25ºC (68º to 77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Advice for the Patient
- Advise patients to remain under the care of a physician while taking Lamivudine Tablets (HBV) and discuss any new symptoms or concurrent medications with their physician.
- Advise patients that Lamivudine Tablets (HBV) is not a cure for hepatitis B, that the long-term treatment benefits of Lamivudine Tablets (HBV) are unknown at this time, and, in particular, that the relationship of initial treatment response to outcomes such as hepatocellular carcinoma and decompensated cirrhosis is unknown [see Dosage and Administration (2.6)].
- Inform patients that deterioration of liver disease has occurred in some cases when treatment was discontinued. Instruct patients to discuss any changes in regimen with their physician [see Warnings and Precautions (5.2)].
- Counsel patients on theim portance of testing for HIV to avoid inappropriate therapy and development of resistant HIV. HIVcounseling and testing should be offered before starting Lamivudine Tablets (HBV) and periodically during therapy.
- Advise patients that Lamivudine Tablets (HBV) contain alower dose of the same active ingredient (lamivudine) as EPIVIR® tablets, EPIVIR® oral solution, COMBIVIR® tablets, EPZICOM® tablets, and TRIZIVIR® tablets. Lamivudine Tablets (HBV) should not be taken concurrently with EPIVIR®, COMBIVIR®, EPZICOM®, or TRIZIVIR® [see Dosage and Administration (2.1), Warnings and Precautions (5.3, 5.4)].
- Advise patients not to take Lamivudine Tablets (HBV) with emtricitabine-containing medicines, such as ATRIPLA®, COMPLERA®, EMTRIVA®, STRIBILD®, or TRUVADA® [see Warnings and Precautions (5.4)].
- Advise patients that treatment with Lamivudine Tablets (HBV) has not been shown to reduce the risk of transmission of HBV to others through sexual contact or blood contamination [see Use in Specific Populations (8.1)].
- Instruct patients to avoid doing things that can spread HBV infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
All registered trademarks are the property of their respective owners.
APOTEX INC.
LAMIVUDINE TABLETS (HBV), 100 mg
Manufactured by: Manufactured for:
Apotex Inc. Apotex Corp.
Totonto, Ontario Weston, Florida
Canada M9L 1T9 33326
Revised: December 2013
Revision: 2
PATIENT INFORMATION
Lamivudine Tablets (HBV), 100 mg
Rx Only
Please read this information before you start taking Lamivudine Tablets (HBV). Re-read it each time you get your prescription, in case some information has changed. This information does not take the place of careful discussions with your doctor when you start this medication and at checkups. Stay under a doctor’s care when you take Lamivudine Tablets (HBV) and do not change or stop treatment without first talking with your doctor.
What is Lamivudine Tablets (HBV)?
Lamivudine Tablets (HBV) is the name of a product that contains lamivudine, a drug used to treat chronic hepatitis B in patients with actively growing virus and liver inflammation. Hepatitis B can cause damage to cells in the liver. Eventually, this can scar the liver.
The lamivudine in Lamivudine Tablets (HBV) can reduce the ability of the hepatitis B virus to multiply and infect new liver cells. It may help to lower the amount of hepatitis B virus in your body.
Lamivudine Tablets (HBV) contain a lower dose of lamivudine than the dose in EPIVIR®, COMBIVIR®, EPZICOM®, and TRIZIVIR®.
Why should I consider HIV testing before starting treatment with Lamivudine Tablets (HBV)?
Your doctor or healthcare provider should offer you counseling and testing for HIV infection (sometimes called the AIDS virus) before treatment for hepatitis B is started with Lamivudine Tablets (HBV), and periodically during treatment. Lamivudine Tablets (HBV) contain a lower dose of the medicine than other lamivudine-containing drugs, such as EPIVIR, COMBIVIR, EPZICOM, and TRIZIVIR which are used to treat HIV. Treatment with Lamivudine Tablets (HBV) in HIV-infected patients may cause the HIV virus to be less treatable with lamivudine and some other drugs.
If I am HIV-positive, can I take Lamivudine Tablets (HBV)?
People who have both chronic hepatitis B and HIV should not take Lamivudine Tablets (HBV). Lamivudine Tablets (HBV) contain a lower dose of the same drug (lamivudine) as EPIVIR Tablets, EPIVIR Oral Solution, COMBIVIR Tablets, EPZICOM Tablets, and TRIZIVIR Tablets. If you have both hepatitis B and HIV, make sure that your doctor or healthcare provider is aware that you have both infections. If you are prescribed lamivudine as part of your combination treatment for HIV, you should use only the products and doses that are intended for treatment of HIV infection, because the lower dose of lamivudine in Lamivudine Tablets (HBV) could cause the HIV virus to be less responsive to treatment. If you are planning to change your HIV treatment to a regimen that does not include EPIVIR, COMBIVIR, EPZICOM, or TRIZIVIR, you should first discuss this change with your doctor or healthcare provider.
Does Lamivudine Tablets (HBV) cure hepatitis B infection?
Lamivudine Tablets (HBV) is not a cure for hepatitis B. In studies comparing lamivudine 100 mg with placebo (an inactive sugar pill) for 1 year, more people treated with lamivudine 100 mg had reductions in liver inflammation. It is not known whether Lamivudine Tablets (HBV) will reduce the risk of getting liver cancer or cirrhosis that may be caused by the hepatitis B virus.
In studies, some patients developed hepatitis B viruses that are resistant to Lamivudine Tablets (HBV). These patients generally had less benefit from treatment with Lamivudine Tablets (HBV). Some patients have had worsening of hepatitis after resistant virus appears. The long-term importance of a resistant virus is not known.
What happens if I stop taking Lamivudine Tablets (HBV)?
After stopping treatment with Lamivudine Tablets (HBV), some patients have had symptoms or blood tests showing that their hepatitis has gotten worse. Therefore, your doctor should check your health, which may include blood tests, for at least several months after stopping treatment with Lamivudine Tablets (HBV). Tell your doctor right away about any new or unusual symptoms that you notice after stopping treatment.
Who should not take Lamivudine Tablets (HBV)?
You should not take Lamivudine Tablets (HBV) if you have or may have HIV infection (sometimes called the AIDS virus). Lamivudine Tablets (HBV) does not contain an appropriate dose of lamivudine for treatment of HIV infection, and using Lamivudine Tablets (HBV) could cause the HIV virus to become less treatable with lamivudine and some other drugs.
You should not take Lamivudine Tablets (HBV) if you are also taking EPIVIR, COMBIVIR, EPZICOM, or TRIZIVIR. These drugs all contain lamivudine.
You should not take Lamivudine Tablets (HBV) if you have had an allergic reaction to lamivudine.
Lamivudine Tablets (HBV) has not been studied in children less than 2 years old.
Can pregnant women and nursing mothers take Lamivudine Tablets (HBV)?
There are no studies of Lamivudine Tablets (HBV) in pregnant women. If you are pregnant or if you become pregnant while taking Lamivudine Tablets (HBV), notify your doctor or healthcare provider immediately.
Lamivudine Tablets (HBV) has not been shown to prevent the spread of the hepatitis B virus from mother to infant.
It is not known whether lamivudine is passed to the infant in breast milk. If there is lamivudine in the breast milk, this could cause side effects in nursing infants. Mothers should not breastfeed while taking Lamivudine Tablets (HBV) or other forms of lamivudine.
How should I take Lamivudine Tablets (HBV)?
Your doctor will tell you how much Lamivudine Tablets (HBV) to take. The usual dose is 1 Lamivudine Tablet (HBV) 100 mg orally (by mouth) once a day. Your doctor may prescribe a lower dose if you have problems with your kidneys. Lamivudine Tablets (HBV) may be taken with food or on an empty stomach. To help you remember to take your Lamivudine Tablets (HBV) as prescribed, you should try to take Lamivudine Tablets (HBV) at the same time each day. You must not skip doses or stop treatment without first talking with your doctor or healthcare provider.
If you miss your regular time for taking your dose, but then remember it during that same day, take your missed dose immediately. Then, take your next dose at the regularly scheduled time the following day. Do not take 2 doses of Lamivudine Tablets (HBV) at once to make up for missing a dose. If you are not sure what to do if you miss taking your medication, check with your doctor or healthcare provider for further instructions.
Lamivudine Tablets (HBV) can usually be taken with many other medications; however, be sure to tell your doctor or healthcare provider about all medications (including over-the-counter and prescription drugs) that you are taking. Lamivudine Tablets (HBV) contain a lower dose of the same drug (lamivudine) as EPIVIR Tablets, EPIVIR Oral Solution, COMBIVIR Tablets, EPZICOM Tablets, and TRIZIVIR Tablets; therefore, Lamivudine Tablets 100 mg should not be taken together with EPIVIR, COMBIVIR, EPZICOM, or TRIZIVIR.
You should talk to your doctor about any changes in your treatment.
What are the possible side effects of Lamivudine Tablets (HBV)?
You should stay under the care of a doctor during treatment so you can be checked for possible serious side effects. Serious side effects such as inflammation of the pancreas can occur with Lamivudine Tablets (HBV). Lactic acid buildup in the body and an enlarged liver have been reported with Lamivudine Tablets (HBV); this is not common but can result in death.
Hepatitis B virus sometimes becomes resistant to Lamivudine Tablets (HBV) during treatment, and some people have had tests showing that their hepatitis was getting worse around the time the virus became resistant. Some people also have worsening of hepatitis after stopping Lamivudine Tablets (HBV). You should discuss any change in treatment with your doctor.
In studies, the most common side effects seen during treatment with Lamivudine Tablets (HBV) were ear, nose, and throat infections; malaise and fatigue (feeling tired and run down); headache; abdominal discomfort and pain; nausea and vomiting; diarrhea; muscle pain; sore throat; joint pain; fever or chills; and skin rash.
This list of possible side effects is not complete. Your doctor or pharmacist can discuss with you a more complete list of possible side effects with Lamivudine Tablets (HBV). Talk to your doctor right away about any side effects or other unusual symptoms that occur when taking Lamivudine Tablets (HBV).
Does Lamivudine Tablets (HBV) reduce the risk of passing hepatitis B to others?
No, Lamivudine Tablets (HBV) has not been shown to reduce the risk of passing hepatitis B to others through sexual contact or exposure to infected blood. Lamivudine Tablets (HBV) also has not been shown to reduce the risk of a mother passing hepatitis B to her baby.
What previous or current medical problems or conditions should I discuss with my doctor or healthcare provider?
Talk to your doctor or healthcare provider if:
- You have HIV infection.
- You are pregnant or if you become pregnant while taking Lamivudine Tablets (HBV).
- You are breastfeeding.
Also talk to your doctor or healthcare provider about:
- Problems with your blood counts.
- Problems with your muscles.
- Problems with your kidneys.
- Problems with your pancreas.
- Any side effects or unusual symptoms during treatment.
How should I store Lamivudine Tablets (HBV)?
Lamivudine Tablets (HBV) should be stored at room temperature. They do not require refrigeration. Keep Lamivudine Tablets (HBV) and all medicines out of the reach of children.
Other Information
This medication is prescribed for a particular condition. Do not use it for any other condition or give it to anybody else.
For more complete information about Lamivudine Tablets (HBV) ask your doctor or pharmacist. You can also ask to read the longer information leaflet that is written for health professionals.
Keep Lamivudine Tablets (HBV) and all medicines out of the reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
EPIVIR®, EPIVIR® Oral Solution, COMBIVIR®, EPZICOM®, and TRIZIVIR® are registered trademarks of their respective owners and are not trademarks of Apotex Inc. The makers of these brands are not affiliated with and do not endorse Apotex Inc. or its products.
APOTEX INC.
LAMIVUDINE TABLETS (HBV), 100 mg
Manufactured by:
Apotex Inc.
Toronto, Ontario
Canada M9L 1T9
Manufactured for:
Apotex Corp.
Weston, Florida
33326
Revised: August 2011
Revision: 1
Repackaged by:
KAISER FOUNDATION HOSPITALS
Livermore, CA 94551
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 100 MG LABEL
APOTEX CORP.
Repackaged By: KAISER FOUNDATION HOSPITALS
Lamivudine Tablets (HBV)
Equivalent to 100 mg lamivudine
Rx only
NDC 0179-0152-70
Box of 30 Unit DoseTablets

LamivudineLamivudine TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||