AMOREPACIFIC
LANEÍGE MYSTIC VEIL FOUNDATION SPF33 PA++
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
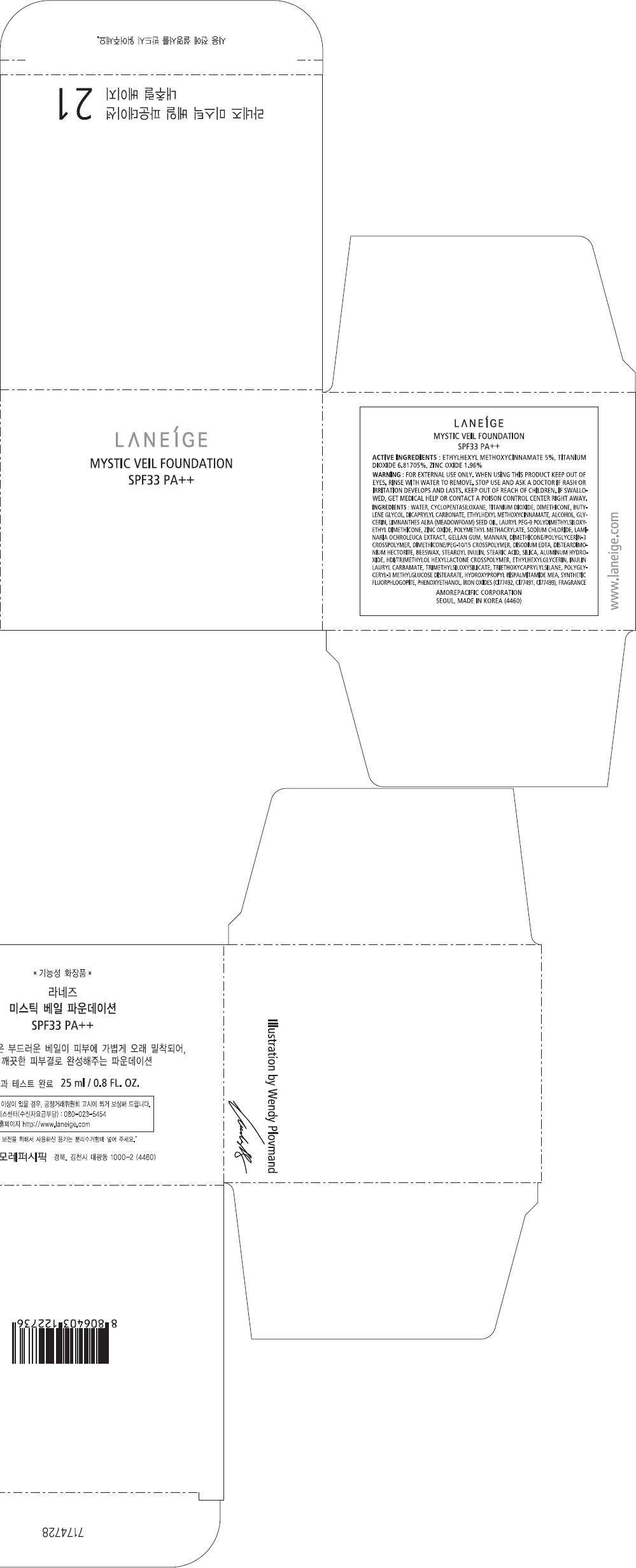
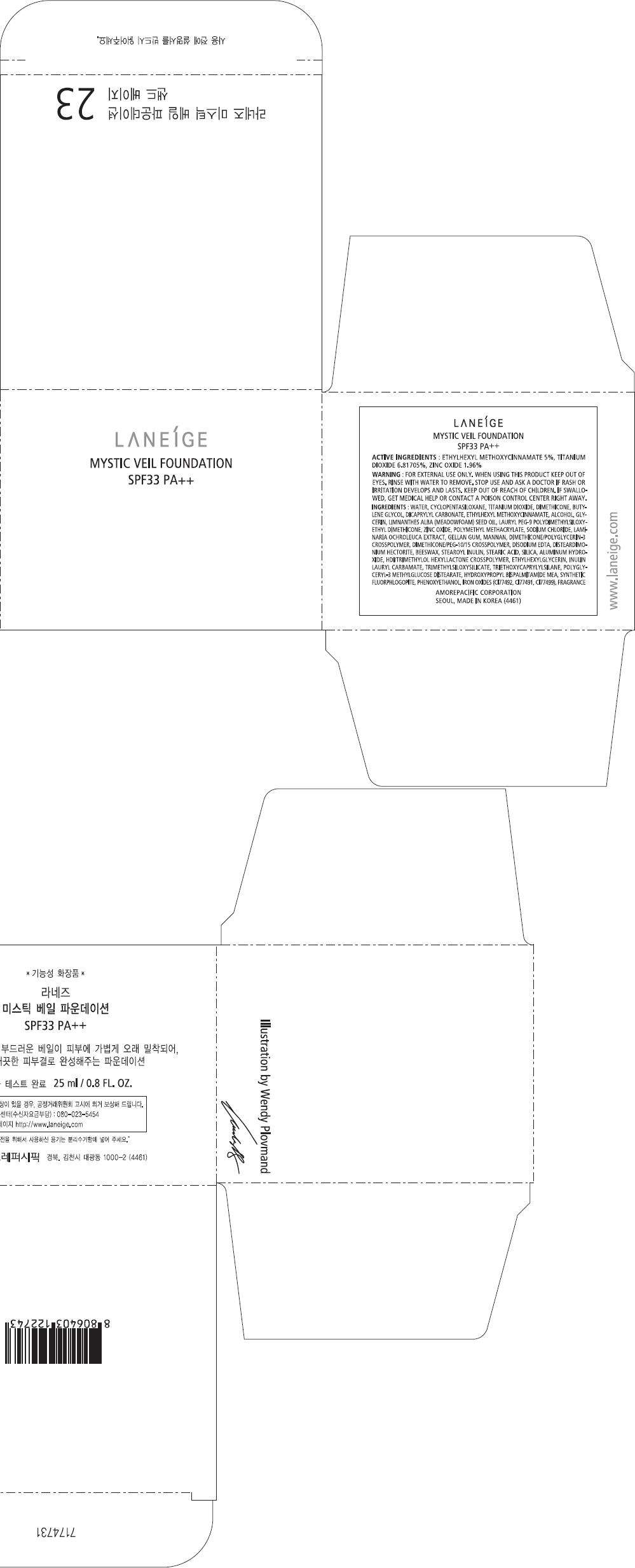
ACTIVE INGREDIENTS
ETHYLHEXYL METHOXYCINNAMATE 5%, TITANIUM DIOXIDE 6.81705%, ZINC OXIDE 1.96%
WARNING
FOR EXTERNAL USE ONLY. WHEN USING THIS PRODUCT KEEP OUT OF EYES. RINSE WITH WATER TO REMOVE.
STOP USE AND ASK A DOCTOR IF RASH OR IRRITATION DEVELOPS AND LASTS.
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
INGREDIENTS
WATER, CYCLOPENTASILOXANE, TITANIUM DIOXIDE, DIMETHICONE, BUTYLENE GLYCOL, DICAPRYLYL CARBONATE, ETHYLHEXYL METHOXYCINNAMATE, ALCOHOL, GLYCERIN, LIMNANTHES ALBA (MEADOWFOAM) SEED OIL, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, ZINC OXIDE, POLYMETHYL METHACRYLATE, SODIUM CHLORIDE, LAMINARIA OCHROLEUCA EXTRACT, GELLAN GUM, MANNAN, DIMETHICONE/POLYGLYCERIN-3 CROSSPOLYMER, DIMETHICONE/PEG-10/15 CROSSPOLYMER, DISODIUM EDTA, DISTEARDIMONIUM HECTORITE, BEESWAX, STEAROYL INULIN, STEARIC ACID, SILICA, ALUMINUM HYDROXIDE, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, ETHYLHEXYLGLYCERIN, INULIN LAURYL CARBAMATE, TRIMETHYLSILOXYSILICATE, TRIETHOXYCAPRYLYLSILANE, POLYGLYCERYL-3 METHYLGLUCOSE DISTEARATE, HYDROXYPROPYL BISPALMITAMIDE MEA, SYNTHETIC FLUORPHLOGOPITE, PHENOXYETHANOL, IRON OXIDES (CI77492, CI77491, CI77499), FRAGRANCE
www.laneige.com
PRINCIPAL DISPLAY PANEL - 21 Carton
LANEÍGE
MYSTIC VEIL FOUNDATION
SPF33 PA++
PRINCIPAL DISPLAY PANEL - 23 Carton
LANEÍGE
MYSTIC VEIL FOUNDATION
SPF33 PA++
LANEIGE MYSTIC VEIL FOUNDATION
Octinoxate, TITANIUM DIOXIDE, and ZINC OXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:43419-744 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:43419-744-60 |
25 in 1 JAR |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-11-01 |
|
|
LANEIGE MYSTIC VEIL FOUNDATION
Octinoxate, TITANIUM DIOXIDE, and ZINC OXIDE CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:43419-745 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:43419-745-61 |
25 in 1 JAR |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-11-01 |
|
|

