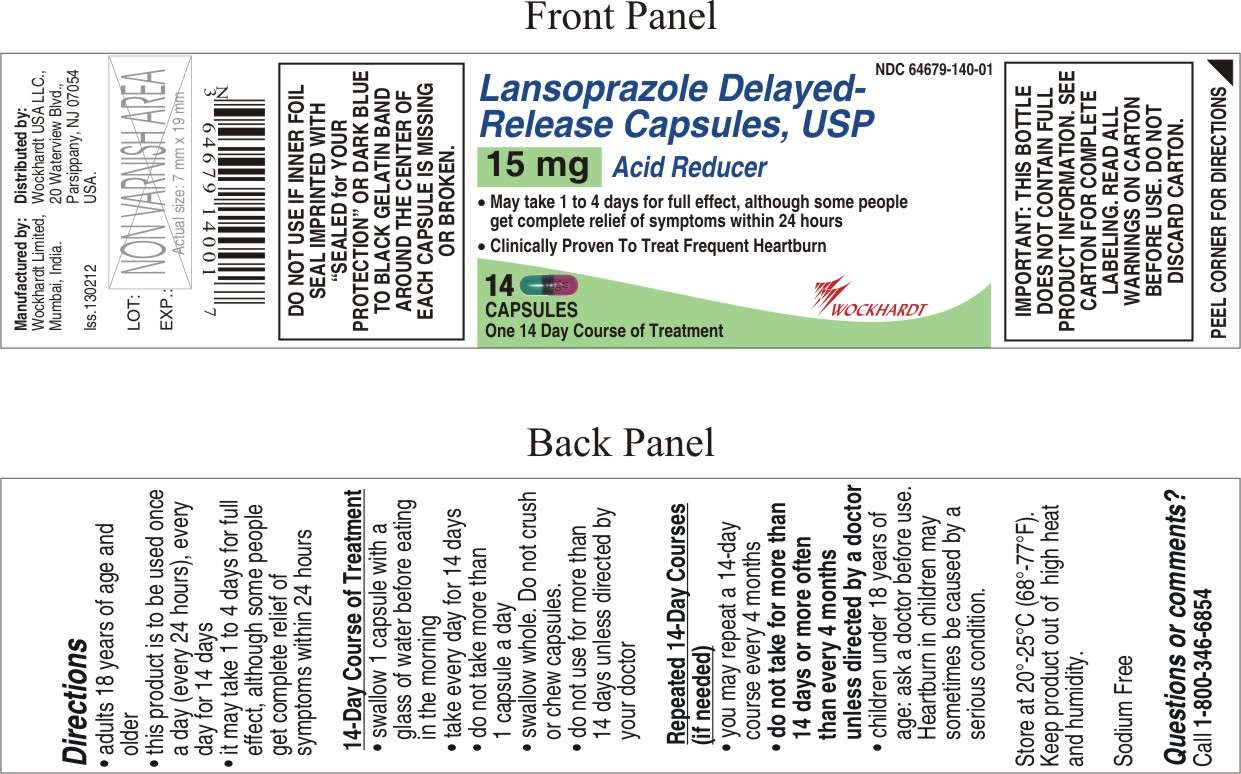
Lansoprazole
Wockhardt Limited
Wockhardt Limited
Lansoprazole Delayed-Release Capsules USP, 15 mg
FULL PRESCRIBING INFORMATION: CONTENTS*
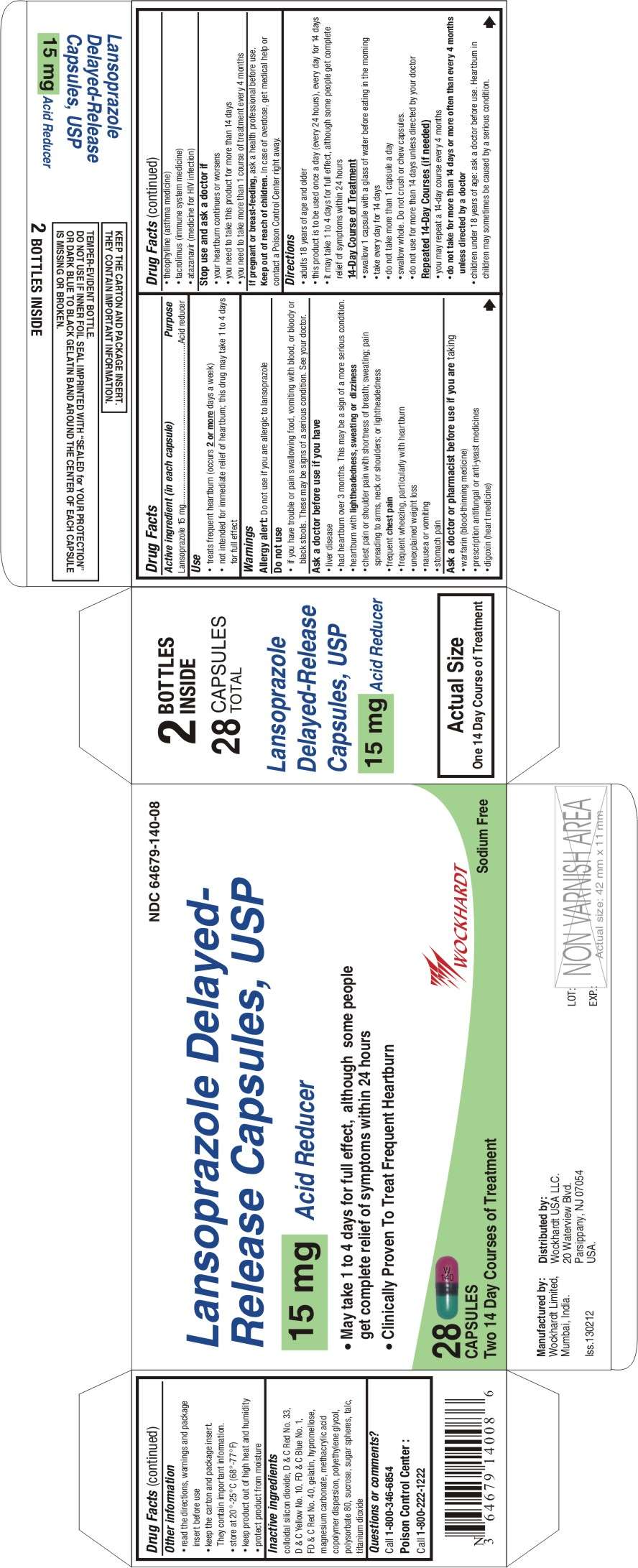
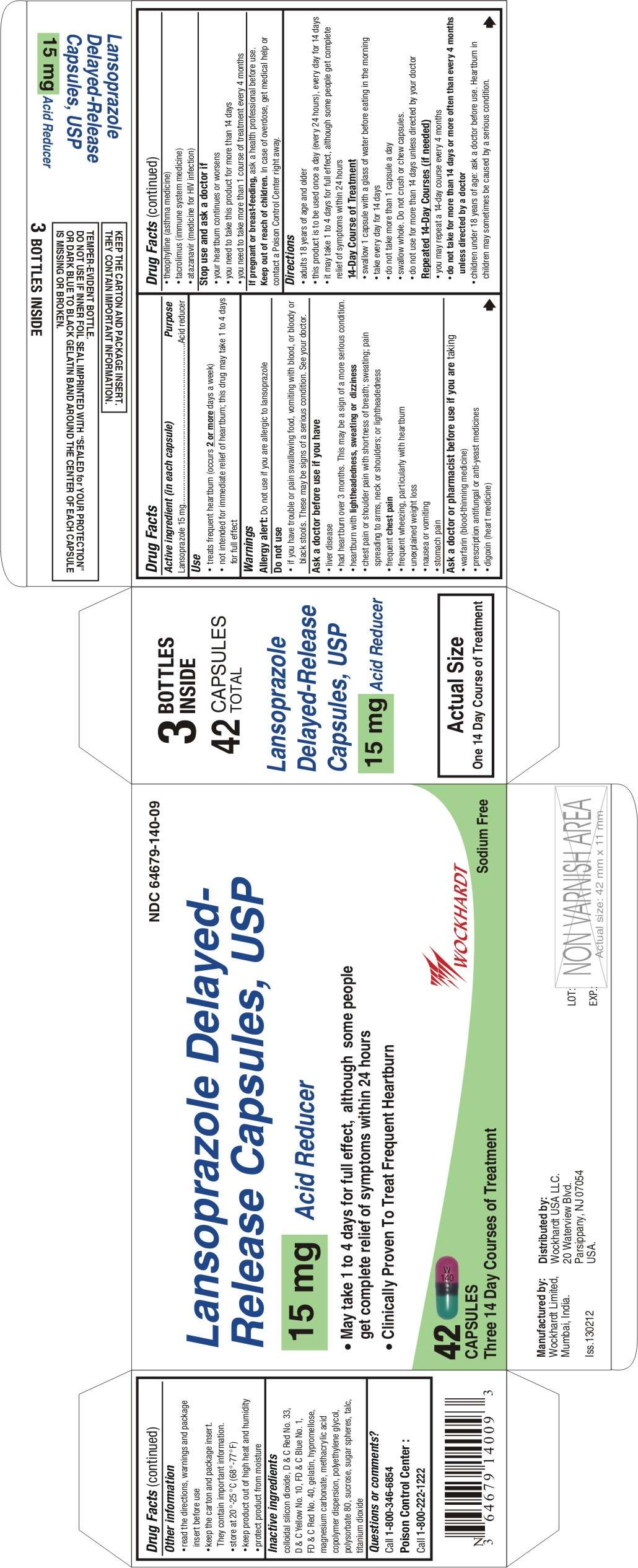
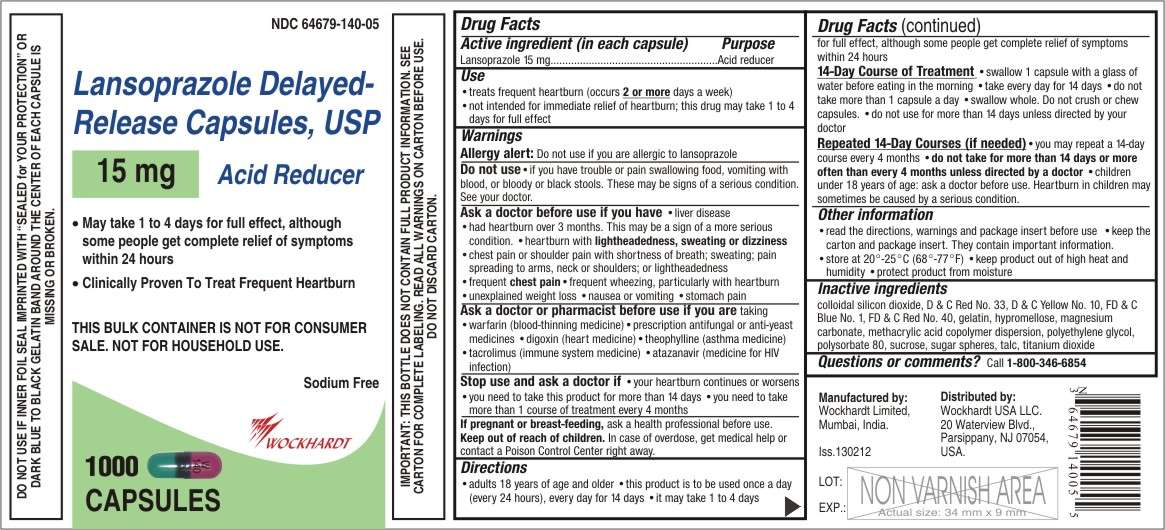
- Active ingredient (in each capsule)
- Purpose
- Use
- WARNINGS
- Keep out of reach of children
- Directions
- Lansoprazole Other information
- Inactive ingredients
- Questions or comments?
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Active ingredient (in each capsule)
Lansoprazole 15 mg
Purpose
Acid reducer
Use
- treats frequent heartburn (occurs 2 or more days a week)
- not intended for immediate relief of heartburn; this drug may take 1 to 4 days for full effect
WARNINGS
Allergy alert: Do not use if you are allergic to lansoprazole
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- liver disease
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Ask a doctor or pharmacist before use if you are taking
- warfarin (blood-thinning medicine)
- prescription antifungal or anti-yeast medicines
- digoxin (heart medicine)
- theophylline (asthma medicine)
- tacrolimus (immune system medicine)
- atazanavir (medicine for HIV infection)
Stop use and ask a doctor if
- your heartburn continues or worsens
- you need to take this product for more than 14 days
- you need to take more than 1 course of treatment every 4 months
If pregnant or breast-feeding
ask a health professional before use.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- adults 18 years of age and older
- this product is to be used once a day (every 24 hours), every day for 14 days
- it may take 1 to 4 days for full effect, although some people get complete relief of symptoms within 24 hours
14-Day Course of Treatment
- swallow 1 capsule with a glass of water before eating in the morning
- take every day for 14 days
- do not take more than 1 capsule a day
- swallow whole. Do not crush or chew capsules.
- do not use for more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
- you may repeat a 14-day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
Lansoprazole Other information
- read the directions, warnings and package insert before use
- keep the carton and package insert. They contain important information.
- store at 20°-25°C (68°-77°F)
- keep product out of high heat and humidity
- protect product from moisture
Inactive ingredients
colloidal silicon dioxide, D & C Red No. 33, D & C Yellow No. 10, FD & C Blue No. 1, FD & C Red No. 40, gelatin, hypromellose, magnesium carbonate, methacrylic acid copolymer dispersion, polyethylene glycol, polysorbate 80, sucrose, sugar spheres, talc, titanium dioxide
Questions or comments?
Call 1-800-346-6854
Poison Control Center:
Call 1-800-222-1222
Manufactured by:
Wockhardt Limited
Mumbai, India.
Distributed by:
Wockhardt USA LLC.
20 Waterview Blvd.
Parsippany, NJ 07054
USA.
Iss.130212
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

LansoprazoleLansoprazole CAPSULE, DELAYED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||