Ventura Corporation LTD
L'BEL Couleur Luxe Rouge Amplifier XP amplifyig lipstick SPF 15
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
|
Active Ingredients
|
Purpose
|
| Octinoxate 4.7%, |
Sunscreen |
| Oxybenzone 0.9 % |
Sunscreen |
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15 Uses
Warnings
-
Skin Cancer / Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
-
Do not use on damaged or broken skin.
-
When using this product keep out of eyes. Rinse with water to remove.
-
Stop use and ask a doctor if rash occurs.
-
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Children under 6 months of age: Ask a doctor.
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15 Other information
- Protect the product in this container from excessive heat and direct sun.
Inactive ingredients
Isostearyl neopentanoate, diisopropyl dimer dilinoleate, mica, methyl hydrogenated rosinate, polyethylene, castor isostearate beeswax succinate, hydrogenated polycyclopentadiene, ozokerite, silica, copernicia cerifera (carnauba) wax, euphorbia cerifera (candelilla) wax, ethylhexyl palmitate, isododecane, hydrogenated polyisobutene, paraffin, polyglyceryl-3 diisostearate, glyceryl isostearate, isostearyl alcohol, dipalmitoyl hydroxyproline, ceramide 3, butyrospermum parkii (shea) butter, beta-sitosterol, propylparaben, dimethicone, luffa cylindrica seed oil, silica silylate, methylparaben, disteardimonium hectorite, fragrance, peg-40 hydrogenated castor oil, polysorbate 80, ppg-26-buteth-26, bht, trimethylsiloxysilicate, hedera helix (ivy) leaf/stem extract, retinyl palmitate, tocopheryl acetate, bioflavonoids, allantoin, ascorbyl palmitate.
May contain: mica, bismuth oxychloride, red 7 lake, manganese violet, iron oxides, titanium dioxide, yellow 5 lake, red 21 lake, red 27 lake, red 6 lake, calcium sodium borosilicate, iron oxides, talc, blue 1 lake, carmine, c9-15 fluoroalcohol phosphate, lauroyl lysine.
PUERTO RICO: Distributed by Ventura Corporation, Ltd. San Juan, Puerto Rico 00926.
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (MARRON ILLIMITÉ) - BROWN
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL
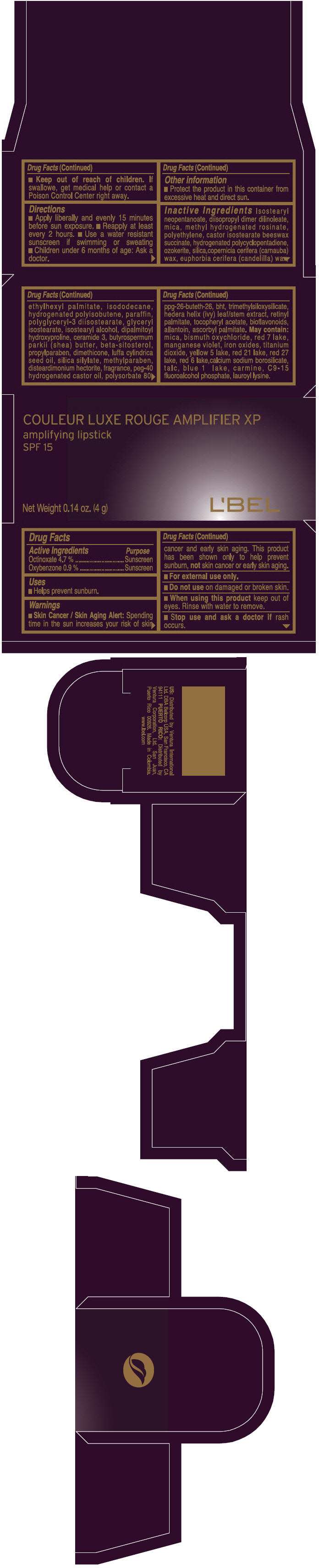
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (CHOCOLAT MAJESTIC) - BROWN
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (CORAIL MAXIMUM) - ORANGE
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (FUCSIA SPLENDIDE) - MAGENTA
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL
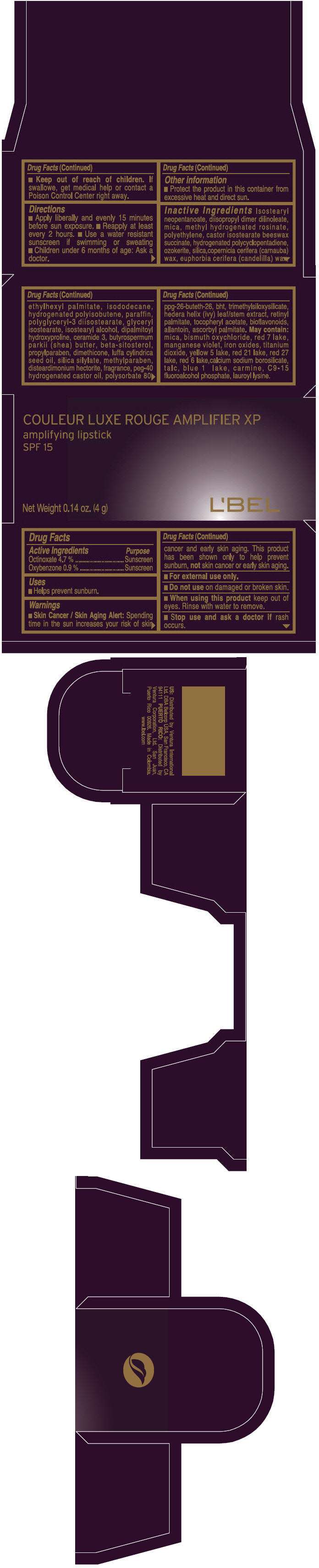
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROUGE PRODIGEUX) - RED
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL
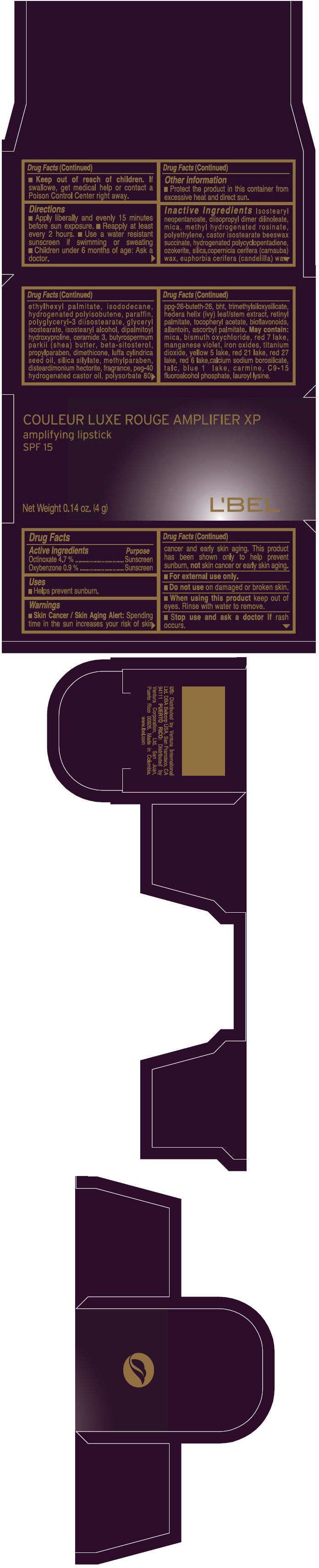
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROUGE GRANDOISE) - RED
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSE CHAMPAGNE) - PINK
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSE D'AMOUR) - PINK
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROUGE RUBI) - RED
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (SAUMON ESSENTIEL) - PINK
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (BOURGOGNE VIF) - BROWN
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (VIOLET POURPRE) - PURPLE
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ORANGE DÉLIRANT) - ORANGE
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSE FEMME) - PINK
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROUGE INTENSE) - RED
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL
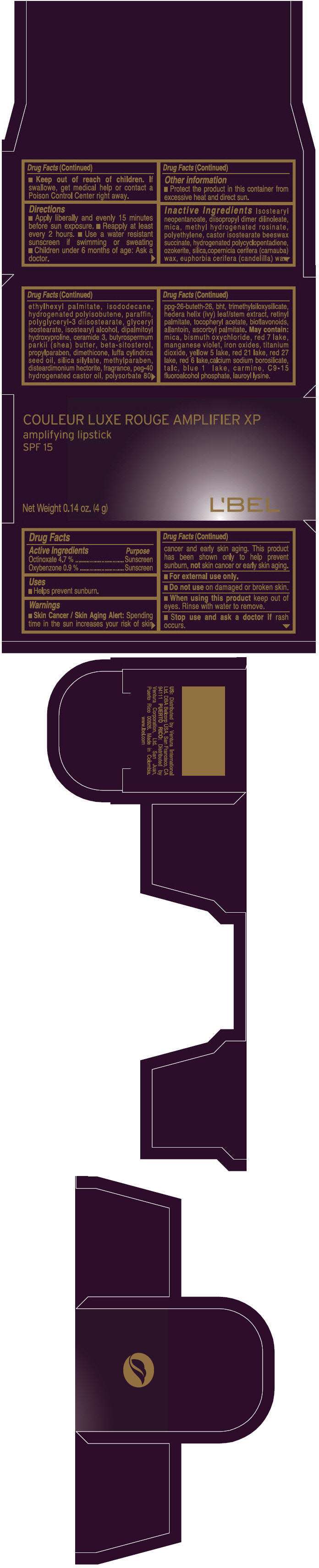
PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (DORÉ CHOCOLAT) - BROWN
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (FUCSIA DÉSIR) - MAGENTA
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (MER DE ROSES) - PINK
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROUGE PROVOCATION) - RED
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSE TENTATION) - PINK
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSE DÉLICAT) - PINK
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (CARMINE) - RED
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (NOBLESSE) - RED
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (MALBEC) - BROWN
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (AMANDIER) - BROWN
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (SOBRIETE) - BROWN
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (FIANCEE) - PINK
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROMANCE) - PINK
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSE FUCSIA) - MAGENTA
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (MIEL ECHANTER) - ORANGE
COULEUR LUXE ROUGE AMPLIFIER XP
amplifying lipstick
SPF 15
Net Weight 0.14 oz. (4 g)
L'BEL

LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-410 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-410-01 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-410-02 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-411 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-411-03 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-411-04 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-412 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-412-05 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-412-06 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-413 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-413-07 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-413-08 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-414 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-414-09 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-414-10 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-415 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-415-11 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-415-12 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-416 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-416-13 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-416-14 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-417 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-417-15 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-417-16 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-418 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-418-17 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-418-18 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-419 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-419-19 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-419-20 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-420 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-420-21 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-420-22 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-115 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-115-23 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-115-24 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-116 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-116-25 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-116-26 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-117 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-117-27 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-117-28 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-118 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-118-29 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-118-30 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-119 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-119-31 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-119-32 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-146 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-146-33 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-146-34 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-149 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-149-35 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-149-36 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-151 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-151-37 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-151-38 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-158 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-158-39 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-158-40 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-159 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-159-41 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-159-42 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-160 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-160-41 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-160-42 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-161 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-161-41 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-161-42 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-162 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-162-41 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-162-42 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-163 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-163-41 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-163-42 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-164 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-164-41 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-164-42 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-165 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-165-41 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-165-42 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-166 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-166-41 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-166-42 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-167 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-167-41 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-167-42 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|
LBEL Couleur Luxe Rouge Amplifier XP amplifying SPF 15
Octinoxate and Oxybenzone LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:13537-168 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.047 g
|
|
OXYBENZONE OXYBENZONE |
|
0.009 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:13537-168-41 |
4 in 1 TUBE |
|
|
|
2 |
NDC:13537-168-42 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2014-01-27 |
|
|





























