LBEL
L'BEL PARIS HYDRA CALME
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- LBEL Uses
- Warnings
- Directions
- LBEL Other information
- Inactive ingredients
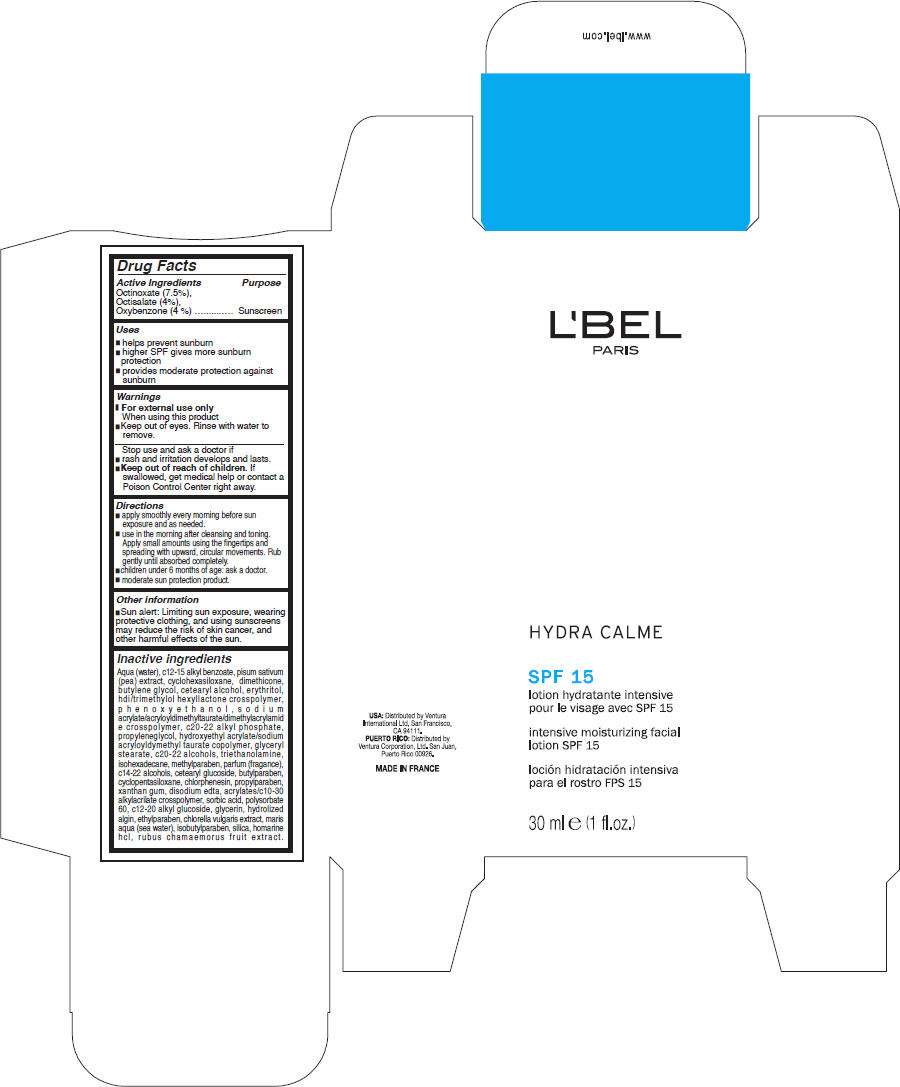
- PRINCIPAL DISPLAY PANEL - 30 ml Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredients
Octinoxate (7.5%), Octisalate (4%), Oxybenzone (4 %)
Purpose
Sunscreen
LBEL Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
- provides moderate protection against sunburn
Warnings
- For external use only
When using this product
- Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash and irritation develops and lasts.
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply smoothly every morning before sun exposure and as needed.
- use in the morning after cleansing and toning. Apply small amounts using the fingertips and spreading with upward, circular movements. Rub gently until absorbed completely.
- children under 6 months of age: ask a doctor.
- moderate sun protection product.
LBEL Other information
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risk of skin cancer, and other harmful effects of the sun.
Inactive ingredients
Aqua (water), c12-15 alkyl benzoate, pisum sativum (pea) extract, cyclohexasiloxane, dimethicone, butylene glycol, cetearyl alcohol, erythritol, hdi/trimethylol hexyllactone crosspolymer, phenoxyethanol, sodium acrylate/acryloyldimethyltaurate/dimethylacrylamide crosspolymer, c20-22 alkyl phosphate, propyleneglycol, hydroxyethyl acrylate/sodium acryloyldymethyl taurate copolymer, glyceryl stearate, c20-22 alcohols, triethanolamine, isohexadecane, methylparaben, parfum (fragance), c14-22 alcohols, cetearyl glucoside, butylparaben, cyclopentasiloxane, chlorphenesin, propylparaben, xanthan gum, disodium edta, acrylates/c10-30 alkylacrilate crosspolymer, sorbic acid, polysorbate 60, c12-20 alkyl glucoside, glycerin, hydrolized algin, ethylparaben, chlorella vulgaris extract, maris aqua (sea water), isobutylparaben, silica, homarine hcl, rubus chamaemorus fruit extract.
USA: Distributed by Ventura
International Ltd, San Francisco,
CA 94111.
PRINCIPAL DISPLAY PANEL - 30 ml Carton
L'BEL
PARIS
HYDRA CALME
SPF 15
intensive moisturizing facial
lotion SPF 15
30 ml e (1 fl.oz.)
LBELOctinoxate, Octisalate, and Oxybenzone LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
LBELOctinoxate, Octisalate, and Oxybenzone LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||