Leflunomide
Leflunomide Tablets USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- LEFLUNOMIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- LEFLUNOMIDE INDICATIONS AND USAGE
- LEFLUNOMIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LEFLUNOMIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- LEFLUNOMIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
BOXED WARNING
CONTRAINDICATIONS AND WARNINGS
Pregnancy
Pregnancy must be excluded before the start of treatment with leflunomide. Leflunomide is contraindicated in pregnant women or women of childbearing potential who are not using reliable contraception (see CONTRAINDICATIONS and WARNINGS.) Pregnancy must be avoided during leflunomide treatment or prior to the completion of the drug elimination procedure after leflunomide treatment.
Hepatotoxicity
Severe liver injury, including fatal liver failure, has been reported in some patients treated with leflunomide. Patients with pre-existing acute or chronic liver disease, or those with serum alanine aminotransferase (ALT) >2xULN before initiating treatment, should not be treated with leflunomide. Use caution when leflunomide is given with other potentially hepatotoxic drugs.
Monitoring of ALT levels is recommended at least monthly for six months after starting leflunomide, and thereafter every 6 to 8 weeks. If ALT elevation > 3 fold ULN occurs, interrupt leflunomide therapy while investigating the probable cause of the ALT elevation by close observation and additional tests. If likely leflunomide-induced, start cholestyramine washout and monitor liver tests weekly until normalized. If leflunomide-induced liver injury is unlikely because some other probable cause has been found, resumption of leflunomide therapy may be considered. (SEE WARNINGS – HEPATOTOXICITY).
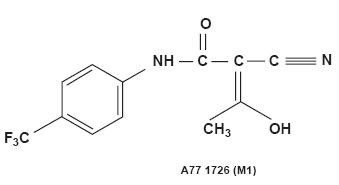
LEFLUNOMIDE DESCRIPTION
129322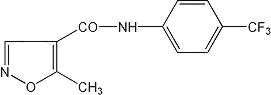
CLINICAL PHARMACOLOGY
Mechanism of Action
in vivo in vitro
Pharmacokinetics
in vivo
|
Table 1. Pharmacokinetic Parameters for M1 after Administration of Leflunomide at Doses of 5, 10, and 25 mg/day for 24 Weeks to Patients (n=54) with Rheumatoid Arthritis (Mean ± SD) (Study YU204)
|
|||
| Maintenance (Loading) Dose |
|||
| Parameter |
5 mg (50 mg) |
10 mg (100 mg) |
25 mg (100 mg) |
| C24 (Day1) (mcg/mL)1
|
4 ± 0.6 |
8.4 ± 2.1 |
8.5± 2.2 |
| C24 (ss) (mcg/mL)2
|
8.8 ± 2.9 |
18 ± 9.6 |
63 ± 36 |
| t1/2(DAYS) |
15 ± 3 |
14 ± 5 |
18 ± 9 |
|
1 Concentration at 24 hours after loading dose 2 Concentration at 24 hours after maintenance doses at steady state |
|||
In vivo in vitro
in vivo PRECAUTIONS - General - Need for Drug Elimination
Gender: in vivo
Age: in vivo CLINICAL PHARMACOLOGY – Special Populations – Pediatrics
Smoking:
Chronic Renal Insufficiency:
Hepatic Insufficiency:
Pediatrics: Table 2
|
Table 2: Population Pharmacokinetic Estimate of M1 Clearance Following Oral Administration of Leflunomide in Pediatric Patients with Polyarticular Course JRA Mean
±SD [Range]
|
||
| N |
Body Weight (kg)
|
CL (mL/h)
|
| 10 |
<20
|
18 ± 9.8 [6.8 to 37]
|
| 30 |
20 to 40
|
18 ± 9.5 [4.2 to 43]
|
| 33 |
>40
|
26 ± 16 [9.7 to 93.6]
|
In vivo
In vitro In vitro in vitroPRECAUTIONS – Drug Interactions).
Methotrexate:
PRECAUTIONS – Drug Interactions – Hepatotoxic Drugs
Rifampin:
CLINICAL STUDIES
A. ADULTS
Table 3
Table 3: Withdrawals in US301
| n (%) patients |
|||
| Leflunomide 190 |
Placebo 128 |
Methotrexate 190 |
|
| Withdrawals in Year – 1 | |||
| Lack of efficacy | 33 (17.4) |
70 (54.7) |
50 (26.3) |
| Safety | 44 (23.2) |
12 (9.4) |
22 (11.6) |
| Other1 |
15 (7.9)
|
10 (7.8)
|
17 (9)
|
| Total | 92 (48.4) |
92 (71.9) |
89 (46.8) |
| Patients entering Year 2 | 98 |
36 |
101 |
| Withdrawals in Year – 2 | |||
| Lack of efficacy | 4 (4.1) |
1 (2.8) |
4 (4) |
| Safety | 8 (8.2) |
0 (0) |
10 (9.9) |
| Other1 |
3 (3.1)
|
8 (22.2)
|
7 (6.9)
|
| Total | 15 (15.3) |
9 (25) |
21 (20.8) |
1
Of the 168 patients who completed 12 months of treatment in MN301 and MN303, 146 patients (87%) entered a 1-year extension study of double blind active treatment (MN305; 60 leflunomide, 60 Sulfasalazine, 26 placebo / sulfasalazine). Patients continued on the same daily dosage of leflunomide or sulfasalazine that they had been taking at the completion of MN301/303. A total of 121 patients (53 leflunomide, 47 sulfasalazine, 21 placebo/ sulfasalazine) completed the 2 years of double-blind treatment.
Table 4
Table 4: Withdrawals in study MN301/303/305
| n (%) patients |
|||
| Leflunomide 133 |
Placebo 92 |
Sulfasalazine 133 |
|
| Withdrawals in MN301 (Mo 0 to 6) | |||
| Lack of efficacy | 10(7.5) |
29(31.5) |
14(10.5) |
| Safety | 19(14.3) |
6(6.5) |
25(18.8) |
| Other1 |
8(6)
|
6(6.5)
|
11(8.3)
|
| Total | 37(27.8) |
41(44.6) |
50(37.6) |
| Patients entering MN303 | 80 |
76 |
|
| Withdrawals in MN303 (Mo 7 to 12) | |||
| Lack of efficacy | 4(5) |
2(2.6) |
|
| Safety | 2(2.5) |
5(6.6) |
|
| Other1 |
3(3.8)
|
1(1.3)
|
|
| Total | 9(11.3) |
8(10.5) |
|
| Patients entering MN305 | 60 |
60 |
|
| Withdrawals in MN305 (Mo 13 to 24) | |||
| Lack of efficacy | 0(0) |
3(5) |
|
| Safety | 6(10) |
8(13.3) |
|
| Other1 |
1(1.7)
|
2(3.3)
|
|
| Total | 7(11.7) |
13(21.7) |
|
1
Table 5
Table 5: Withdrawals in MN302/304
| n (%) patients |
||
| Leflunomide 501 |
Methotrexate 498 |
|
| Withdrawals in MN302 (Year – 1) | ||
| Lack of efficacy | 37 (7.4) |
15 (3) |
| Safety | 98 (19.6) |
79 (15.9) |
| Other1 | 17 (3.4) |
17 (3.4) |
| Total |
152 (30.3)
|
111 (22.3)
|
| Patients entering MN304 | 292 |
320 |
| Withdrawals in MN304 (Year – 2) | ||
| Lack of efficacy | 13 (4.5) |
9 (2.8) |
| Safety | 11(3.8) |
22 (6.9) |
| Other1 | 12 (4.1) |
12 (3.8) |
| Total |
36 (12.3)
|
43 (13.4)
|
1
Clinical Trial Data
1. Signs and symptoms Rheumatoid Arthritis
The ACR20 Responder at Endpoint rates are shown in Figure 1. Leflunomide was statistically significantly superior to placebo in reducing the signs and symptoms of RA by the primary efficacy analysis, ACR20 Responder at Endpoint, in study US301 (at the primary 12 months endpoint) and MN301 (at 6 month endpoint). ACR20 Responder at Endpoint rates with leflunomide treatment were consistent across the 6 and 12 month studies (41to 49%). No consistent differences were demonstrated between leflunomide and methotrexate or between leflunomide and sulfasalazine. Leflunomide treatment effect was evident by 1 month, stabilized by 3 to 6 months, and continued throughout the course of treatment as shown in Figure 2.
Figure 1
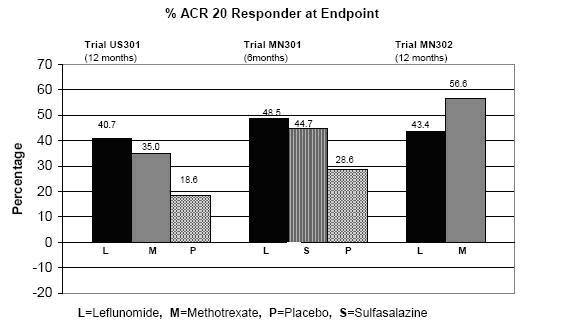
| Comparisons
|
95%Confidence Interval
|
p Value
|
|
| US301 | Leflunomide vs. Placebo |
(12, 32) |
<0.0001 |
| Methotrexate vs. Placebo |
(8, 30) |
<0.0001 |
|
| Leflunomide vs. Methotrexate |
(-4, 16) |
NS |
|
| MN301 | Leflunomide vs. Placebo |
(7, 33) |
0.0026 |
| Sulfasalazine vs. Placebo |
(4, 29) |
0.0121 |
|
| Leflunomide vs. Sulfasalazine |
(-8, 16) |
NS |
|
| MN302 | Leflunomide vs. Methotrexate |
(-19, -7) |
<0.0001 |
Figure 2
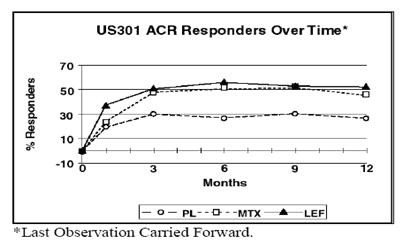
Table 6Table 7
| Table 6 Summary of ACR Response Rates* | |||
| Study and Treatment Group | ACR20 |
ACR50 |
ACR70 |
| Placebo-Controlled Studies | |||
| US301 (12 months) | |||
| Leflunomide(n=178)† | 52.2‡
|
34.3‡ |
20.2‡
|
| Placebo (n = 118)† | 26.3 |
7.6 |
4.2 |
| Methotrexate (n = 180)† | 45.6 |
22.8 |
9.4 |
| MN301 (6 months) | |||
| Leflunomide (n = 130)† | 54.6‡
|
33.1‡
|
10§
|
| Placebo (n = 91)† | 28.6 |
14.3 |
2.2 |
| Sulfasalazine (n = 132)† | 56.8 |
30.3 |
7.6 |
| Non-PlaceboActive-Controlled Studies | |||
| MN302 (12 months) | |||
| Leflunomide (n = 495)† | 51.1 |
31.1 |
9.9 |
| Methotrexate (n = 489)† | 65.2 |
43.8 |
16.4 |
|
*Intent to treat (ITT) analysis using last observation carried forward (LOCF) technique for patients who discontinued early. †N is the number of ITT patients for whom adequate data were available to calculate the indicated rates. ‡ p<0.001 leflunomide vs placebo §p<0.02 leflunomide vs placebo |
|||
Table 7
| Table 7 Mean Change in the Components of the ACR Responder Index* | ||||||||
| Components | Placebo-Controlled Studies |
Non-placebo Controlled Study |
||||||
|
US301 (12 months) |
MN301 Non-US (6 months) |
MN302 Non-US (12 months) |
||||||
| Leflu-nomide |
Metho-trexate |
Placebo |
Leflu-nomide |
Sulfa-salazine |
Placebo |
Leflu-nomide |
Metho-trexate |
|
| Tender joint count1 | -7.7 |
-6.6 |
-3.0 |
-9.7 |
-8.1 |
-4.3 |
-8.3 |
-9.7 |
| Swollen joint count1 | -5.7 |
-5.4 |
-2.9 |
-7.2 |
-6.2 |
-3.4 |
-6.8 |
-9 |
| Patient global assessment2 | -2.1 |
-1.5 |
0.1 |
-2.8 |
-2.6 |
-0.9 |
-2.3 |
-3 |
| Physician global assessment2 | -2.8 |
-2.4 |
-1.0 |
-2.7 |
-2.5 |
-0.8 |
-2.3 |
-3.1 |
| Physical Function / disability (MHAQ / HAQ) | -0.29 |
-0.15 |
0.07 |
-0.50 |
-0.29 |
-0.04 |
-0.37 |
-0.44 |
| Pain intensity2 | -2.2 |
-1.7 |
-0.5 |
-2.7 |
-2.0 |
-0.9 |
-2.1 |
-2.9 |
| Erythrocyte Sedimentation rate | -6.26 |
-6.48 |
2.56 |
-7.48 |
-16.56 |
3.44 |
-10.12 |
-22.18 |
| C-reactive protein | -0.62 |
-0.50 |
0.47 |
-2.26 |
-1.19 |
0.16 |
-1.86 |
-2.45 |
| Not included in the ACR Responder Index | ||||||||
| Morning Stiffness (min) | -101.4 |
-88.7 |
14.7 |
-93.0 |
-42.4 |
-6.8 |
-63.7 |
-86.6 |
|
*Last Observation Carried Forward; Negative Change Indicates Improvement 1Based on 28 joint count 2Visual Analog Scale - 0=Best; 10 = Worst |
||||||||
2. Maintenance of effect
After completing 12 months of treatment, patients continuing on study treatment were evaluated for an additional 12 months of double-blind treatment (total treatment period of 2 years) in studies US301, MN305, and MN304. ACR Responder rates at 12 months were maintained over 2 years in most patients continuing a second year of treatment.
3. Inhibition of structural damage
Figure 3
Figure 3
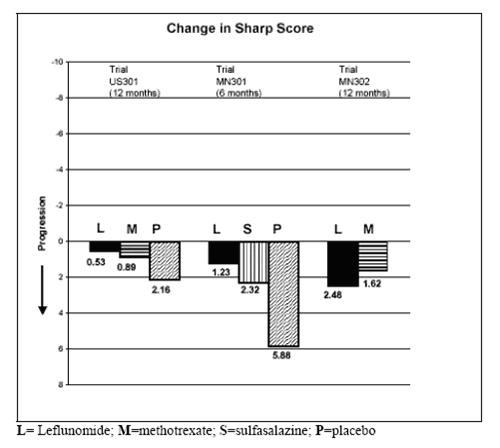
| Comparisons | %Confidence Interval | p Value | |
| US301 | Leflunomide vs. Placebo | (-4, -1.1) |
0.0007 |
| Methotrexate vs. Placebo | (-2.6, -0.2) |
0.0196 |
|
| Leflunomide vs. Methotrexate | (-2.3, 0) |
0.0499 |
|
| MN301 | Leflunomide vs. Placebo | (-6.2, -1.8) |
0.0004 |
| Sulfasalazine vs. Placebo | (-6.9, 0) |
0.0484 |
|
| Leflunomide vs. Sulfasalazine | (3.3, 1.2) |
NS |
|
| MN302 | Leflunomide vs. Methotrexate | (-2.2, 7.4) |
NS |
4. Improvement in physical function
Figure 4
Figure 4
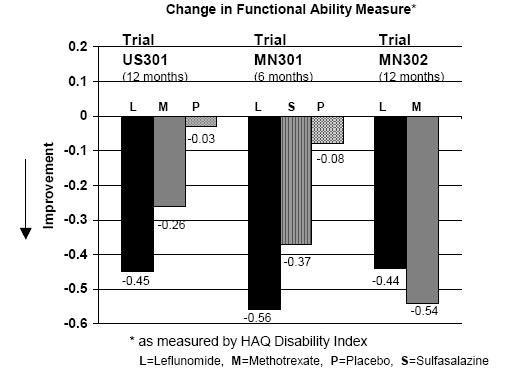
|
Comparisons
|
95%Confidence Interval
|
p Value
|
|
| US 301 | Leflunomide vs. Placebo | (-0.58, -0.29) |
0.0001 |
| Leflunomide vs. Methotrexate |
(-0.34, -0.07) |
0.0026 |
|
| MN301 | Leflunomide vs. Placebo |
(-0.67, -0.36) |
<0.0001 |
| Leflunomide vs. Sulfasalazine |
(-0.33, -0.03) |
0.0163 |
|
| MN 302 | Leflunomide vs. Methotrexate |
(0.01, 0.16) |
0.0221 |
Maintenance of effect
B. PEDIATRICS
Pharmacokinetics – Pediatrics.
LEFLUNOMIDE INDICATIONS AND USAGE
CLINICAL STUDIES
PRECAUTIONS – Drug Interactions – NSAIDs WARNINGS – Immunosuppression Potential / Bone Marrow Suppression
LEFLUNOMIDE CONTRAINDICATIONS
WARNINGS
Hepatotoxicity
PRECAUTIONS - General - Need for Drug Elimination
| Table 8 Liver Enzyme Elevations >3-fold Upper Limits of Normal (ULN) | ||||||||
|
US301
|
MN301
|
MN302*
|
||||||
|
LEF
|
PL
|
MTX
|
LEF
|
PL
|
SSZ
|
LEF
|
MTX
|
|
|
ALT (SGPT) >3-fold ULN (n%) Reversed to £2-fold ULN |
8 (4.4) 8 |
3 (2.5) 3 |
5 (2.7) 5 |
2 (1.5) 2 |
1 (1.1) 1 |
2 (1.5) 2 |
13 (2.6) 12 |
83 (16.7) 82 |
|
Timing of Elevation 0 to 3 Months 4 to 6 Months 7 to 9 Months 10 to 12 Months |
6 1 1 - |
1 1 1 - |
1 3 1 - |
2 - - - |
1 - - - |
2 - - - |
7 1 - 5 |
27 34 16 6 |
Immunosuppression Potential / Bone Marrow Suppression
PRECAUTIONS – General – Need for Drug EliminationPneumocystis jiroveci Pneumocystis jiroveci
PRECAUTIONS – General – Need for Drug Elimination
Skin Reactions
PRECAUTIONS – General – Need for Drug Elimination
Malignancy
Use in Women of Childbearing Potential
CONTRAINDICATIONS
Peripheral Neuropathy
Cases of peripheral neuropathy have been reported in patients receiving leflunomide. Most patients recovered after discontinuation of leflunomide, but some patients had persistent symptoms. Age older than 60 years, concomitant neurotoxic medications, and diabetes may increase the risk for peripheral neuropathy. If a patient taking leflunomide develops a peripheral neuropathy, consider discontinuing leflunomide therapy and performing the drug elimination procedure (see WARNINGS – Drug Elimination Procedure).
Drug Elimination Procedure
The following drug elimination procedure is recommended to achieve non-detectable plasma levels (less than 0.02 mg/L or 0.02 mcg/mL) after stopping treatment with leflunomide:
1) Administer cholestyramine 8 grams 3 times daily for 11 days. (The 11 days do not need to be consecutive unless there is a need to lower the plasma level rapidly.)
2) Verify plasma levels less than 0.02 mg/L (0.02 mcg/mL) by two separate tests at least 14 days apart. If plasma levels are higher than 0.02 mg/L, additional cholestyramine treatment should be considered.
Without the drug elimination procedure, it may take up to 2 years to reach plasma M1 metabolite levels less than 0.02 mg/L due to individual variation in drug clearance.
PRECAUTIONS
General
ADVERSE REACTIONSWARNINGS – Drug Elimination Procedure
Information for Patients
- The potential for increased risk of birth defects should be discussed with female patients of childbearing potential. It is recommended that physicians advise women that they may be at increased risk of having a child with birth defects if they are pregnant when taking leflunomide, become pregnant while taking leflunomide, or do not wait to become pregnant until they have stopped taking leflunomide and followed the drug elimination procedure (as described in WARNINGS – Use In Women of Childbearing Potential – Drug Elimination Procedure).
- Patients should be advised of the possibility of rare, serious skin reactions. Patients should be instructed to inform their physicians promptly if they develop a skin rash or mucous membrane lesions.
- Patients should be advised of the potential hepatotoxic effects of leflunomide and of the need for monitoring liver enzymes. Patients should be instructed to notify their physicians if they develop symptoms such as unusual tiredness, abdominal pain or jaundice.
- Patients should be advised that they may develop a lowering of their blood counts and should have frequent hematologic monitoring. This is particularly important for patients who are receiving other immunosuppressive therapy concurrently with leflunomide, who have recently discontinued such therapy before starting treatment with leflunomide, or who have had a history of a significant hematologic abnormality. Patients should be instructed to notify their physicians promptly if they notice symptoms of pancytopenia (such as easy bruising or bleeding, recurrent infections, fever, paleness or unusual tiredness).
- Patients should be informed about the early warning signs of interstitial lung disease and asked to contact their physician as soon as possible if these symptoms appear or worsen during therapy.
Laboratory Tests
WARNINGS – Immunosuppression Potential / Bone Marrow Suppression
At minimum, ALT (SGPT) must be performed at baseline and at least monthly for six months after starting leflunomide, and thereafter every 6 to 8 weeks. In addition, if leflunomide and methotrexate are given concomitantly, ACR guidelines for monitoring methotrexate liver toxicity must be followed with ALT, AST, and serum albumin testing every month. (See WARNINGS – Hepatotoxicity.)
Drug Interactions
PRECAUTIONS – General – Need for Drug Elimination
CLINICAL PHARMACOLOGY
in vitro
in vitro
Carcinogenesis, Mutagenesis, Impairment Of Fertility
No evidence of carcinogenicity was observed in a 2-year bioassay in rats at oral doses of leflunomide up to the maximally tolerated dose of 6 mg/kg (approximately 1/40 the maximum human M1 systemic exposure based on AUC). However, male mice in a 2-year bioassay exhibited an increased incidence in lymphoma at an oral dose of 15 mg/kg, the highest dose studied (1.7 times the human M1 exposure based on AUC). Female mice, in the same study, exhibited a dose-related increased incidence of bronchoalveolar adenomas and carcinomas combined beginning at 1.5 mg/kg (approximately 1/10 the human M1 exposure based on AUC). The significance of the findings in mice relative to the clinical use of leflunomide is not known.
Leflunomide was not mutagenic in the Ames Assay, the Unscheduled DNA Synthesis Assay, or in the HGPRT Gene Mutation Assay. In addition, leflunomide was not clastogenic in the in vivo Mouse Micronucleus Assay nor in the in vivo Cytogenetic Test in Chinese Hamster Bone Marrow Cells. However, 4- trifluoromethylaniline (TFMA), a minor metabolite of leflunomide, was mutagenic in the Ames Assay and in the HGPRT Gene Mutation Assay, and was clastogenic in the in vitro Assay for Chromosome Aberrations in the Chinese Hamster Cells. TFMA was not clastogenic in the in vivo Mouse Micronucleus Assay nor in the in vivo Cytogenetic Test in Chinese Hamster Bone Marrow Cells. Leflunomide had no effect on fertility in either male or female rats at oral doses up to 4 mg/kg (approximately 1/30 the human M1 exposure based on AUC).
Pregnancy
CONTRAINDICATIONSNursing Mothers
Leflunomide should not be used by nursing mothers. It is not known whether leflunomide is excreted in human milk. Many drugs are excreted in human milk, and there is a potential for serious adverse reactions in nursing infants from leflunomide. Therefore, a decision should be made whether to proceed with nursing or to initiate treatment with leflunomide, taking into account the importance of the drug to the mother.
Use in Males
Available information does not suggest that leflunomide would be associated with an increased risk of male-mediated fetal toxicity. However, animal studies to evaluate this specific risk have not been conducted. To minimize any possible risk, men wishing to father a child should consider discontinuing use of leflunomide and taking cholestyramine 8 grams 3 times daily for 11 days.
Pediatric Use
The safety and effectiveness of leflunomide in pediatric patients with polyarticular course juvenile rheumatoid arthritis (JRA) have not been fully evaluated (See CLINICAL STUDIES and ADVERSE REACTIONS).
Geriatric Use
Of the total number of subjects in controlled clinical (Phase III) studies of leflunomide, 234 subjects were 65 years and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. No dosage adjustment is needed in patients over 65.
LEFLUNOMIDE ADVERSE REACTIONS
Table 9| Table 9 Percentage of Patients with Adverse Events ≥3% In Any Leflunomide Treated Group | |||||||
|
All RA
Studies
|
Placebo-Controlled Trials
|
Active-Controlled Trials
|
|||||
| MN 301 and US 301
|
MN 302*
|
||||||
|
LEF (N=1339)1 |
LEF (N=315)
|
PBO (N=210)
|
SSZ (N=133)
|
MTX (N=182)
|
LEF (N=501)
|
MTX (N=498)
|
|
| BODY AS A WHOLE | |||||||
| Allergic Reaction | 2% |
5% |
2% |
0% |
6% |
1% |
2% |
| Asthenia | 3% |
6% |
4% |
5% |
6% |
3% |
3% |
| Flu Syndrome | 2% |
4% |
2% |
0% |
7% |
0% |
0% |
| Infection, upper respiratory | 4% |
0% |
0% |
0% |
0% |
0% |
0% |
| Injury Accident | 5% |
7% |
5% |
3% |
11% |
6% |
7% |
| Pain | 2% |
4% |
2% |
2% |
5% |
1% |
<1% |
| Abdominal Pain | 6% |
5% |
4% |
4% |
8% |
6% |
4% |
| Back Pain | 5% |
6% |
3% |
4% |
9% |
8% |
7% |
| CARDIOVASCULAR | |||||||
| Hypertension2 | 10% |
9% |
4% |
4% |
3% |
10% |
4% |
| -New onset of hypertension | 1% |
<1% |
0% |
2% |
2% |
<1% |
|
| Chest Pain | 2% |
4% |
2% |
2% |
4% |
1% |
2% |
| GASTROINTESTINAL | |||||||
| Anorexia | 3% |
3% |
2% |
5% |
2% |
3% |
3% |
| Diarrhea | 17% |
27% |
12% |
10% |
20% |
22% |
10% |
| Dyspepsia | 5% |
10% |
10% |
9% |
13% |
6% |
7% |
| Gastroenteritis | 3% |
1% |
1% |
0% |
6% |
3% |
3% |
| Abnormal Liver Enzymes | 5% |
10% |
2% |
4% |
10% |
6% |
17% |
| Nausea | 9% |
13% |
11% |
19% |
18% |
13% |
18% |
| GI / Abdominal Pain | 5% |
6% |
4% |
7% |
8% |
8% |
8% |
| Mouth Ulcer | 3% |
5% |
4% |
3% |
10% |
3% |
6% |
| Vomiting | 3% |
5% |
4% |
4% |
3% |
3% |
3% |
| METABOLIC AND NUTRITIONAL | |||||||
| Hypokalemia | 1% |
3% |
1% |
1% |
1% |
1% |
<1% |
| Weight Loss3 | 4% |
2% |
1% |
2% |
0% |
2% |
2% |
| MUSCULO-SKELETAL SYSTEM | |||||||
| Arthralgia | 1% |
4% |
3% |
0% |
9% |
<1% |
1% |
| Leg Cramps | 1% |
4% |
2% |
2% |
6% |
0% |
0% |
| Joint Disorder | 4% |
2% |
2% |
2% |
2% |
8% |
6% |
| Synovitis | 2% |
<1% |
1% |
0% |
2% |
4% |
2% |
| Tenosynovitis | 3% |
2% |
0% |
1% |
2% |
5% |
1% |
| NERVOUS SYSTEM | |||||||
| Dizziness | 4% |
5% |
3% |
6% |
5% |
7% |
6% |
| Headache | 7% |
13% |
11% |
12% |
21% |
10% |
8% |
| Paresthesia | 2% |
3% |
1% |
1% |
2% |
4% |
3% |
| RESPIRATORY SYSTEM | |||||||
| Bronchitis | 7% |
5% |
2% |
4% |
7% |
8% |
7% |
| Increased Cough | 3% |
4% |
5% |
3% |
6% |
5% |
7% |
| Respiratory Infection | 15% |
21% |
21% |
20% |
32% |
27% |
25% |
| Pharyngitis | 3% |
2% |
1% |
2% |
1% |
3% |
3% |
| Pneumonia | 2% |
3% |
0% |
0% |
1% |
2% |
2% |
| Rhinitis | 2% |
5% |
2% |
4% |
3% |
2% |
2% |
| Sinusitis | 2% |
5% |
5% |
0% |
10% |
1% |
1% |
|
SKIN AND
APPENDAGES
|
|||||||
| Alopecia | 10% |
9% |
1% |
6% |
6% |
17% |
10% |
| Eczema | 2% |
1% |
1% |
1% |
1% |
3% |
2% |
| Pruritus | 4% |
5% |
2% |
3% |
2% |
6% |
2% |
| Rash | 10% |
12% |
7% |
11% |
9% |
11% |
10% |
| Dry Skin | 2% |
3% |
2% |
2% |
0% |
3% |
1% |
| UROGENITAL SYSTEM | |||||||
| Urinary Tract Infection | 5% |
5% |
7% |
4% |
2% |
5% |
6% |
| * | Only 10% of patients in MN302 received folate. All patients in US301 received folate; none in MN301 received folate. |
| 1 | Includes all controlled and uncontrolled trials with leflunomide (duration up to 12 months). |
| 2 | Hypertension as a preexisting condition was overrepresented in all leflunomide treatment groups in phase III trials |
| 3 | In a meta-analysis of all phase II and III studies, during the first 6 months in patients receiving leflunomide, 10% lost 10 to 19 lbs (24 cases per 100 patient years) and 2% lost at least 20 lbs (4 cases/100 patient years). Of patients receiving leflunomide, 4% lost 10% of their baseline weight during the first 6 months of treatment. |
Body as a Whole:
Cardiovascular: angina pectoris, migraine, palpitation, tachycardia, varicose vein, vasculitis, vasodilatation;
Gastrointestinal: cholelithiasis, colitis, constipation, esophagitis, flatulence, gastritis, gingivitis, melena, oral moniliasis, pharyngitis, salivary gland enlarged, stomatitis (or aphthous stomatitis), tooth disorder;
Endocrine: diabetes mellitus, hyperthyroidism;
Hemic and Lymphatic System: anemia (including iron deficiency anemia), ecchymosis;
Metabolic and Nutritional: creatine phosphokinase increased, hyperglycemia, hyperlipidemia, peripheral edema;
Musculo-Skeletal System: arthrosis, bone necrosis, bone pain, bursitis, muscle cramps, myalgia, tendon rupture;
Nervous System: anxiety, depression, dry mouth, insomnia, neuralgia, neuritis, sleep disorder, sweating increased, vertigo;
Respiratory System: asthma, dyspnea, epistaxis, lung disorder;
Skin and Appendages: acne, contact dermatitis, fungal dermatitis, hair discoloration, hematoma, herpes simplex, herpes zoster, maculopapular rash, nail disorder, skin discoloration, skin disorder, skin nodule, subcutaneous nodule, ulcer skin;
Special Senses: blurred vision, cataract, conjunctivitis, eye disorder, taste perversion;
Urogenital System: albuminuria, cystitis, dysuria, hematuria, menstrual disorder, prostate disorder, urinary frequency, vaginal moniliasis.
Other less common adverse events seen in clinical trials include: 1 case of anaphylactic reaction occurred in Phase 2 following rechallenge of drug after withdrawal due to rash (rare); urticaria;eosinophilia; transient thrombocytopenia (rare); and leukopenia <2000 WBC/mm3 (rare).
Adverse events during a second year of treatment with leflunomide in clinical trials were consistent with those observed during the first year of treatment and occurred at a similar or lower incidence.
In post-marketing experience, the following have been reported rarely:
Body as a whole: opportunistic infections, severe infections including sepsis that may be fatal;
Gastrointestinal: pancreatitis;
Hematologic: agranulocytosis, leukopenia, neutropenia, pancytopenia, thrombocytopenia;
Hypersensitivity:
Hepatic:
Respiratory:
Nervous system:
Skin and Appendages:
Adverse Reactions (Pediatric Patients)
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
ADVERSE REACTIONS
PRECAUTIONS – General – Need for Drug Elimination
CLINICAL PHARMACOLOGY – Elimination
LEFLUNOMIDE DOSAGE AND ADMINISTRATION
Loading Dose
WARNINGS – Hepatotoxicity
Maintenance Therapy
Monitoring
PRECAUTIONS – Laboratory Tests; WARNINGS – HepatotoxicityWARNINGS – Immunosuppression Potential/Bone Marrow Suppression
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL


Leflunomideleflunomide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Leflunomideleflunomide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||