LETROZOLE
Sun Pharmaceutical Industries Limited
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use letrozole safely and effectively. See full prescribing information for letrozole tablets. Letrozole Tablets, USPInitial U.S. Approval: 1997 RECENT MAJOR CHANGES1.12.2INDICATIONS AND USAGELetrozole tablets are aromatase inhibitors indicated for: Adjuvant treatment of postmenopausal women with hormone receptor positive early breast cancer (1.1) Extended adjuvant treatment of postmenopausal women with early breast cancer who have received prior standard adjuvant tamoxifen therapy (1.2) First and second-line treatment of postmenopausal women with hormone receptor positive or unknown advanced breast cancer (1.3) DOSAGE AND ADMINISTRATIONLetrozole tablets are taken orally without regard to meals (2): Recommended dose: 2.5 mg once daily (2.1) Patients with cirrhosis or severe hepatic impairment: 2.5 mg every other day (2.5, 5.3) DOSAGE FORMS AND STRENGTHS3CONTRAINDICATIONS4WARNINGS AND PRECAUTIONS Decreases in bone mineral density may occur. Consider bone mineral density monitoring (5.1) Increases in total cholesterol may occur. Consider cholesterol monitoring. (5.2) Fatigue, dizziness and somnolence may occur. Exercise caution when operating machinery (5.4) Side Effects6.16.26.36.4To report SUSPECTED ADVERSE REACTIONS, contact CARACO Pharmaceutical Laboratories Ltd. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 LETROZOLE INDICATIONS AND USAGE
- 2 LETROZOLE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 LETROZOLE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 LETROZOLE ADVERSE REACTIONS
- 6.1 Adjuvant Treatment of Early Breast Cancer
- 6.2 Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 24 Months
- 6.3 Updated Analysis, Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 60 Months
- 6.4 First-Line Treatment of Advanced Breast Cancer
- 6.5 Second- Line Treatment of Advanced Breast Cancer
- 6.6 First and Second-Line Treatment of Advanced Breast Cancer
- 6.7 Postmarketing Experience
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 LETROZOLE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 14.1 Updated Adjuvant Treatment of Early Breast Cancer
- 14.2 Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 24 Months
- 14.3 Updated Analyses of Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 60 Months
- 14.4 First-Line Treatment of Advanced Breast Cancer
- 14.5 Second-Line Treatment of Advanced Breast Cancer
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Adjuvant Treatment of Early Breast Cancer
Letrozole
1.2 Extended Adjuvant Treatment of Early Breast Cancer
see Clinical Studies (14.2, 14.3)
1.3 First and Second-Line Treatment of Advanced Breast Cancer
see Clinical Studies (14.4, 14.5)
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Use in Adjuvant Treatment of Early Breast Cancer
In see Clinical Studies (14.1)
2.3 Use in Extended Adjuvant Treatment of Early Breast Cancer
see Clinical Studies (14.2)
2.4 Use in First and Second-Line Treatment of Advanced Breast Cancer
see Clinical Studies (14.4, 14.5)
2.5 Use in Hepatic Impairment
see Warnings and Precautions (5.3)
2.6 Use in Renal Impairment
see Clinical Pharmacology (12.3)
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
see Use in Specific Populations (8.1)
5 WARNINGS AND PRECAUTIONS
5.1 Bone Effects
PSee Adverse reactions (6.1)[see Adverse Reactions (6.2)].
See Adverse Reactions (6.1)[see Adverse Reactions (6.3)].
5.2 Cholesterol
[see Adverse Reactions (6.1)]
5.3 Hepatic Impairment
[see Dosage and Administration (2.5)]
5.4 Fatigue and Dizziness
5.5 Laboratory Test Abnormalities
6 ADVERSE REACTIONS
The most serious adverse reactions from the use of letrozole are:
- Bone effects [see Warnings and Precautions (5.1)]
- Increases in cholesterol [see Warnings and Precautions (5.2)]
6.1 Adjuvant Treatment of Early Breast Cancer
| Grades 1 to 4 | Grades 3 to 4 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse Reaction | Letrozole N=2448 n (%) |
Tamoxifen N=2447 n (%) |
Letrozole N=2448 n (%) |
Tamoxifen N=2447 n (%) |
||||||||
| Note: Cardiovascular (including cerebrovascular and thromboembolic), skeletal and urogenital/endometrial events and second malignancies were collected life-long. All of these events were assumed to be of CTC grade 3 to 5 and were not individually graded. |
||||||||||||
| Pts with any adverse event |
2310 |
(94.4) |
2214 |
(90.5) |
635 |
(25.9) |
604 |
(24.7) |
||||
| Hypercholesterolemia |
1280 |
(52.3) |
700 |
(28.6) |
11 |
(0.4) |
6 |
(0.2) |
||||
| Hot Flashes/Flushes |
821 |
(33.5) |
929 |
(38) |
0 |
- |
0 |
- |
||||
| Arthralgia/Arthritis |
618 |
(25.2) |
501 |
(20.4) |
85 |
(3.5) |
50 |
(2) |
||||
| Night Sweats |
357 |
(14.6) |
426 |
(17.4) |
0 |
- |
0 |
- |
||||
Bone Fractures |
338 |
(13.8) |
257 |
(10.5) |
- |
- |
- |
- |
||||
| Weight Increase |
317 |
(12.9) |
378 |
(15.4) |
27 |
(1.1) |
39 |
(1.6) |
||||
| Nausea |
283 |
(11.6) |
277 |
(11.3) |
6 |
(0.2) |
9 |
(0.4) |
||||
Bone Fractures |
247 |
(10.1) |
174 |
(7.1) |
- |
- |
- |
- |
||||
| Fatigue (Lethargy, Malaise, Asthenia) |
235 |
( 9.6) |
250 |
(10.2) |
6 |
(0.2) |
7 |
(0.3) |
||||
| Myalgia |
217 |
(8.9) |
212 |
(8.7) |
18 |
(0.7) |
14 |
(0.6) |
||||
| Edema |
164 |
(6.7) |
160 |
(6.5) |
3 |
(0.1) |
1 |
(<0.1) |
||||
| Weight Decrease |
140 |
(5.7) |
129 |
(5.3) |
8 |
(0.3) |
5 |
(0.2) |
||||
| Vaginal Bleeding |
128 |
(5.2) |
320 |
(13.1) |
1 |
(<0.1) |
8 |
(0.3) |
||||
| Back Pain |
125 |
(5.1) |
136 |
(5.6) |
7 |
(0.3) |
11 |
(0.4) |
||||
| Osteoporosis NOS |
124 |
(5.1) |
66 |
(2.7) |
10 |
(0.4) |
5 |
(0.2) |
||||
| Bone pain |
123 |
(5) |
109 |
(4.5) |
6 |
(0.2) |
4 |
(0.2) |
||||
| Depression |
119 |
(4.9) |
114 |
(4.7) |
16 |
(0.7) |
14 |
(0.6) |
||||
| Vaginal Irritation |
111 |
(4.5) |
77 |
(3.1) |
2 |
(<0.1) |
2 |
(<0.1) |
||||
| Headache |
105 |
(4.3) |
94 |
(3.8) |
9 |
(0.4) |
5 |
(0.2) |
||||
| Pain in extremity |
103 |
(4.2) |
79 |
(3.2) |
6 |
(0.2) |
4 |
(0.2) |
||||
| Osteopenia |
87 |
(3.6) |
74 |
(3) |
0 |
- |
2 |
(<0.1) |
||||
| Dizziness/Light-Headedness |
84 |
(3.4) |
84 |
(3.4) |
1 |
(<0.1) |
6 |
(0.2) |
||||
| Alopecia |
83 |
(3.4) |
84 |
(3.4) |
0 |
- |
0 |
- |
||||
| Vomiting |
80 |
(3.3) |
80 |
(3.3) |
3 |
(0.1) |
5 |
(0.2) |
||||
| Cataract |
49 |
(2) |
54 |
(2.2) |
16 |
(0.7) |
17 |
(0.7) |
||||
| Constipation |
49 |
(2) |
71 |
(2.9) |
3 |
(0.1) |
1 |
(<0.1) |
||||
| Breast pain |
37 |
(1.5) |
43 |
(1.8) |
1 |
(<0.1) |
0 |
- |
||||
| Anorexia |
20 |
(0.8) |
20 |
(0.8) |
1 |
(<0.1) |
1 |
(<0.1) |
||||
Endometrial Hyperplasia/Cancer  |
11/1909 |
(0.6) |
70/1943 |
(3.6) |
- |
- |
- |
- |
||||
|
Endometrial Proliferation Disorders |
10 |
(0.3) |
71 |
(1.8) |
0 |
- |
14 |
(0.6) |
||||
Endometrial Hyperplasia/Cancer  |
6/1909 |
( 0.3) |
57/1943 |
(2.9) |
- |
- |
- |
- |
||||
| Other Endometrial Disorders |
2 |
(<0.1) |
3 |
(0.1) |
0 |
- |
- |
- |
||||
Myocardial Infarction |
24 |
(1) |
12 |
(0.5) |
- |
- |
- |
- |
||||
Myocardial Infarction |
37 |
(1.5) |
25 |
(1) |
- |
- |
- |
- |
||||
| Myocardial Ischemia |
6 |
(0.2) |
9 |
(0.4) |
- |
- |
- |
- |
||||
Cerebrovascular Accident |
52 |
(2.1) |
46 |
(1.9) |
- |
- |
- |
- |
||||
Cerebrovascular Accident |
70 |
(2.9) |
63 |
(2.6) |
- |
- |
- |
- |
||||
Angina |
26 |
(1.1) |
24 |
(1) |
- |
- |
- |
- |
||||
Angina |
32 |
(1.3) |
31 |
(1.3) |
- |
- |
- |
- |
||||
Thromboembolic Event |
51 |
(2.1) |
89 |
(3.6) |
- |
- |
- |
- |
||||
Thromboembolic Event |
71 |
(2.9) |
111 |
(4.5) |
- |
- |
- |
- |
||||
Other Cardiovascular |
260 |
(10.6) |
256 |
(10.5) |
- |
- |
- |
- |
||||
Other Cardiovascular |
312 |
(12.7) |
337 |
(13.8) |
- |
- |
- |
- |
||||
Second Malignancies |
53 |
(2.2) |
78 |
(3.2) |
- |
- |
- |
- |
||||
Second Malignancies |
102 |
(4.2) |
119 |
(4.9) |
- |
- |
- |
- |
||||
P
6.2 Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 24 Months
| Number (%) of Patients with Grade 1 to 4 Adverse Reaction | Number (%) of Patients with Grade 3 to 4 Adverse Reaction | |||
|---|---|---|---|---|
| Letrozole N=2563 | Placebo N=2573 |
Letrozole N=2563 | Placebo N=2573 |
|
|
Any Adverse Reaction
|
2232 (87.1) |
2174 (84.5) |
419 (16.3) |
389 (15.1) |
|
Vascular Disorders
|
1375 (53.6) |
1230 (47.8) |
59 (2.3) |
74 (2.9) |
| Flushing |
1273 (49.7) |
1114 (43.3) |
3 (0.1) |
0 - |
|
General Disorders
|
1154 (45) |
1090 (42.4) |
30 (1.2) |
28 (1.1) |
| Asthenia |
862 (33.6) |
826 (32.1) |
16 (0.6) |
7 (0.3) |
| Edema NOS |
471 (18.4) |
416 (16.2) |
4 (0.2) |
3 (0.1) |
|
Musculoskeletal Disorders
|
978 (38.2) |
836 (32.5) |
71 (2.8) |
50 (1.9) |
| Arthralgia |
565 (22) |
465 (18.1) |
25 (1) |
20 (0.8) |
| Arthritis NOS |
173 (6.7) |
124 (4.8) |
10 (0.4) |
5 (0.2) |
| Myalgia |
171 (6.7) |
122 (4.7) |
8 (0.3) |
6 (0.2) |
| Back Pain |
129 (5) |
112 (4.4) |
8 (0.3) |
7 (0.3) |
|
Nervous System Disorders
|
863 (33.7) |
819 (31.8) |
65 (2.5) |
58 (2.3) |
| Headache |
516 (20.1) |
508 (19.7) |
18 (0.7) |
17 (0.7) |
| Dizziness |
363 (14.2) |
342 (13.3) |
9 (0.4) |
6 (0.2) |
|
Skin Disorders
|
830 (32.4) |
787 (30.6) |
17 (0.7) |
16 (0.6) |
| Sweating Increased |
619 (24.2) |
577 (22.4) |
1 (<0.1) |
0 - |
|
Gastrointestinal Disorders
|
725 (28.3) |
731 (28.4) |
43 (1.7) |
42 (1.6) |
| Constipation |
290 (11.3) |
304 (11.8) |
6 (0.2) |
2 (<0.1) |
| Nausea |
221 (8.6) |
212 (8.2) |
3 (0.1) |
10 (0.4) |
| Diarrhea NOS |
128 (5) |
143 (5.6) |
12 (0.5) |
8 (0.3) |
|
Metabolic Disorders
|
551 (21.5) |
537 (20.9) |
24 (0.9) |
32 (1.2) |
| Hypercholesterolemia |
401 (15.6) |
398 (15.5) |
2 (<0.1) |
5 (0.2) |
|
Reproductive Disorders
|
303 (11.8) |
357 (13.9) |
9 (0.4) |
8 (0.3) |
| Vaginal Hemorrhage |
123 (4.8) |
171 (6.6) |
2 (<0.1) |
5 (0.2) |
| Vulvovaginal Dryness |
137 (5.3) |
127 (4.9) |
0 - |
0 - |
|
Psychiatric Disorders
|
320 (12.5) |
276 (10.7) |
21 (0.8) |
16 (0.6) |
| Insomnia |
149 (5.8) |
120 (4.7) |
2 (<0.1) |
2 (<0.1) |
|
Respiratory Disorders
|
279 (10.9) |
260 (10.1) |
30 (1.2) |
28 (1.1) |
| Dyspnea |
140 (5.5) |
137 (5.3) |
21 (0.8) |
18 (0.7) |
|
Investigations
|
184 (7.2) |
147 (5.7) |
13 (0.5) |
13 (0.5) |
|
Infections and Infestations
|
166 (6.5) |
163 (6.3) |
40 (1.6) |
33 (1.3) |
|
Renal Disorders
|
130 (5.1) |
100 (3.9) |
12 (0.5) |
6 (0.2) |
Bone Sub-study: see Warnings and Precautions (5.1)
Lipid Sub-study: [see Warnings and Precautions (5.2)]
6.3 Updated Analysis, Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 60 Months
see Adverse Reactions (6.2)
[see Warnings and Precautions (5.2)]
6.4 First-Line Treatment of Advanced Breast Cancer
| Adverse Reaction | Letrozole 2.5 mg (N=455) % |
Tamoxifen 20 mg (N=455) % |
|---|---|---|
|
General Disorders
|
||
| Fatigue |
13 |
13 |
| Chest Pain |
8 |
9 |
| Edema Peripheral |
5 |
6 |
| Pain NOS |
5 |
7 |
| Weakness |
6 |
4 |
|
Investigations
|
||
| Weight Decreased |
7 |
5 |
|
Vascular Disorders
|
||
| Hot Flushes |
19 |
16 |
| Hypertension |
8 |
4 |
|
Gastrointestinal Disorders
|
||
| Nausea |
17 |
17 |
| Constipation |
10 |
11 |
| Diarrhea |
8 |
4 |
| Vomiting |
7 |
8 |
|
Infections/Infestations
|
||
| Influenza |
6 |
4 |
| Urinary Tract Infection NOS |
6 |
3 |
|
Injury, Poisoning and Procedural Complications
|
||
| Post-Mastectomy Lymphedema |
7 |
7 |
|
Metabolism and Nutrition Disorders
|
||
| Anorexia |
4 |
6 |
|
Musculoskeletal and Connective Tissue Disorders
|
||
| Bone Pain |
22 |
21 |
| Back Pain |
18 |
19 |
| Arthralgia |
16 |
15 |
| Pain in Limb |
10 |
8 |
|
Nervous System Disorders
|
||
| Headache NOS |
8 |
7 |
|
Psychiatric Disorders
|
||
| Insomnia |
7 |
4 |
|
Reproductive System and Breast Disorders
|
||
| Breast Pain |
7 |
7 |
|
Respiratory, Thoracic and Mediastinal Disorders
|
||
| Dyspnea |
18 |
17 |
| Cough |
13 |
13 |
| Chest Wall Pain |
6 |
6 |
6.5 Second- Line Treatment of Advanced Breast Cancer
| Adverse Reaction |
Pooled Letrozole 2.5 mg (N=359) % |
Pooled Letrozole 0.5 mg (N=380) % |
Megestrol Acetate 160 mg (N=189) % |
Aminoglutethimide 500 mg (N=178) % |
|---|---|---|---|---|
|
Body as a Whole
|
||||
| Fatigue |
8 |
6 |
11 |
3 |
| Chest Pain |
6 |
3 |
7 |
3 |
Peripheral Edema |
5 |
5 |
8 |
3 |
| Asthenia |
4 |
5 |
4 |
5 |
| Weight Increase |
2 |
2 |
9 |
3 |
|
Cardiovascular
|
||||
| Hypertension |
5 |
7 |
5 |
6 |
|
Digestive System
|
||||
| Nausea |
13 |
15 |
9 |
14 |
| Vomiting |
7 |
7 |
5 |
9 |
| Constipation |
6 |
7 |
9 |
7 |
| Diarrhea |
6 |
5 |
3 |
4 |
| Pain-Abdominal |
6 |
5 |
9 |
8 |
| Anorexia |
5 |
3 |
5 |
5 |
| Dyspepsia |
3 |
4 |
6 |
5 |
|
Infections/Infestations
|
||||
| Viral Infection |
6 |
5 |
6 |
3 |
|
Lab Abnormality
|
||||
| Hypercholesterolemia |
3 |
3 |
0 |
6 |
|
Musculoskeletal System
|
||||
Musculoskeletal |
21 |
22 |
30 |
14 |
| Arthralgia |
8 |
8 |
8 |
3 |
|
Nervous System
|
||||
| Headache |
9 |
12 |
9 |
7 |
| Somnolence |
3 |
2 |
2 |
9 |
| Dizziness |
3 |
5 |
7 |
3 |
|
Respiratory System
|
||||
| Dyspnea |
7 |
9 |
16 |
5 |
| Coughing |
6 |
5 |
7 |
5 |
|
Skin and Appendages
|
||||
| Hot Flushes |
6 |
5 |
4 |
3 |
Rash |
5 |
4 |
3 |
12 |
| Pruritus |
1 |
2 |
5 |
3 |
6.6 First and Second-Line Treatment of Advanced Breast Cancer
6.7 Postmarketing Experience
7 DRUG INTERACTIONS
Tamoxifen
Cimetidine
Warfarin
Other anticancer agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category Xsee Contraindications (4)
2
2 2 2 see Nonclinical Toxicology (13.2)
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
2 2
11 DESCRIPTION
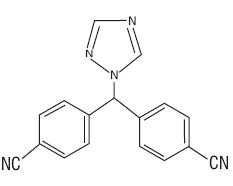
17115
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
Absorption and Distribution:
Metabolism and Excretion:
Pediatric, Geriatric and Race:
Renal Impairment:
Hepatic Impairment:
see Dosage and Administration (2.5)
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
2 0-12hr0-24hr 2 0-24hr
in vitro testsE.coliin vitroin vivo
2
Letrozole
13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology: 2 2
2
14 CLINICAL STUDIES
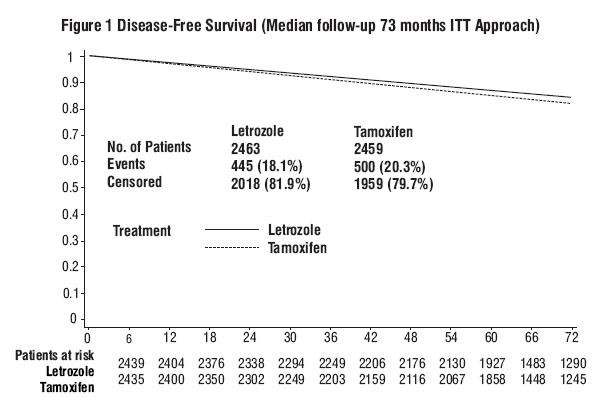
14.1 Updated Adjuvant Treatment of Early Breast Cancer
P
| Characteristic | Primary Core Analysis (PCA) | Monotherapy Arms Analysis (MAA) | ||
|---|---|---|---|---|
| Letrozole N=4003 n (%) |
Tamoxifen N=4007 n (%) |
Letrozole N=2463 n (%) |
Tamoxifen N=2459 n (%) |
|
| Age (median, years) |
61 |
61 |
61 |
61 |
| Age range (years) |
38 to 89 |
39 to 90 |
38 to 88 |
39 to 90 |
| Hormone receptor status (%) |
||||
| ER+ and/or PgR+ |
99.7 |
99.7 |
99.7 |
99.7 |
| Both unknown |
0.3 |
0.3 |
0.3 |
0.3 |
| Nodal status (%) |
||||
| Node negative |
52 |
52 |
50 |
52 |
| Node positive |
41 |
41 |
43 |
41 |
| Nodal status unknown |
7 |
7 |
7 |
7 |
| Prior adjuvant chemotherapy (%) |
24 |
24 |
24 |
24 |
| Letrozole N=2463 |
Tamoxifen N=2459 |
Hazard ratio (95% CI) |
P |
||||
|---|---|---|---|---|---|---|---|
| Events (%) | 5-year rate | Events (%) | 5-year rate | ||||
| Definition of: |
|||||||
Disease-free survival |
ITT |
445 (18.1) |
87.4 |
500 (20.3) |
84.7 |
0.87 (0.76, 0.99) |
0.03 |
| |
Censor |
445 |
87.4 |
483 |
84.2 |
0.84 (0.73, 0.95) |
|
| 0 positive nodes |
ITT |
165 |
92.2 |
189 |
90.3 |
0.88 (0.72, 1.09) |
|
| 1 to 3 positive nodes |
ITT |
151 |
85.6 |
163 |
83 |
0.85 (0.68, 1.06) |
|
| >=4 positive nodes |
ITT |
123 |
71.2 |
142 |
62.6 |
0.81 (0.64, 1.03) |
|
| Adjuvant chemotherapy |
ITT |
119 |
86.4 |
150 |
80.6 |
0.77 (0.60, 0.98) |
|
| No chemotherapy |
ITT |
326 |
87.8 |
350 |
86.1 |
0.91 (0.78, 1.06) |
|
Systemic DFS |
ITT |
401 |
88.5 |
446 |
86.6 |
0.88 (0.77,1.01) |
|
Time to distant metastasis |
ITT |
257 |
92.4 |
298 |
90.1 |
0.85 (0.72, 1) |
|
| Adjuvant chemotherapy |
ITT |
84 |
- |
109 |
- |
0.75 (0.56-1) |
|
| No chemotherapy |
ITT |
173 |
- |
189 |
- |
0.90 (0.73,1.11) |
|
Distant DFS |
ITT |
385 |
89 |
432 |
87.1 |
0.87 (0.76,1) |
|
| Contralateral breast cancer |
ITT |
34 |
99.2 |
44 |
98.6 |
0.76 (0.49, 1.19) |
|
| Overall survival |
ITT |
303 |
91.8 |
343 |
90.9 |
0.87 (0.75, 1.02) |
|
| |
Censor |
303 |
91.8 |
338 |
90.1 |
0.82 (0.70, 0.96) |
|
| 0 positive nodes |
ITT |
107 |
95.2 |
121 |
94.8 |
0.90 (0.69.1.16) |
|
| 1 to 3 positive nodes |
ITT |
99 |
90.8 |
114 |
90.6 |
0.81(0.62,1.06) |
|
| >=4 positive nodes |
ITT |
92 |
80.2 |
104 |
73.6 |
0.86 (0.65, 1.14) |
|
| Adjuvant chemotherapy |
ITT |
76 |
91.5 |
96 |
88.4 |
0.79 (0.58, 1.06) |
|
| No chemotherapy |
ITT |
227 |
91.9 |
247 |
91.8 |
0.91 (0.76, 1.08) |
|
14.2 Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 24 Months
| Baseline Status | Letrozole N=2582 | Placebo N=2586 |
|---|---|---|
|
Hormone Receptor Status (%)
|
||
| ER+ and/or PgR+ |
98 |
98 |
| Both Unknown |
2 |
2 |
|
Nodal Status (%)
|
||
| Node Negative |
50 |
50 |
| Node Positive |
46 |
46 |
| Nodal Status Unknown |
4 |
4 |
|
Chemotherapy
|
46 |
46 |
| Letrozole N = 2582 |
Placebo N = 2586 |
Hazard Ratio (95% CI) | P-Value | |
|---|---|---|---|---|
| CI = confidence interval for hazard ratio. Hazard ratio of less than 1 indicates difference in favor of letrozole (lesser risk of recurrence); hazard ratio greater than 1 indicates difference in favor of placebo (higher risk of recurrence with letrozole). |
||||
Disease Free Survival (DFS) |
122 (4.7%) |
193 (7.5%) |
0.62 (0.49, 0.78) |
0.00003 |
| Local Breast Recurrence |
9 |
22 |
||
| Local Chest Wall Recurrence |
2 |
8 |
||
| Regional Recurrence |
7 |
4 |
||
| Distant Recurrence |
55 |
92 |
0.61 (0.44 to 0.84) |
0.003 |
| Contralateral Breast Cancer |
19 |
29 |
||
| Deaths Without Recurrence or Contralateral Breast Cancer |
30 |
38 |
||
14.3 Updated Analyses of Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 60 Months
| Letrozole N = 2582 (%) |
Placebo N = 2586 (%) |
Hazard Ratio (95% CI) |
P-Value |
|
|---|---|---|---|---|
Disease Free Survival (DFS) events |
344 (13.3) |
402 (15.5) |
0.89 (0.77, 1.03) |
0.12 |
|
Breast cancer recurrence
(Protocol definition of DFS events  |
209 |
286 |
0.75 (0.63, 0.89) |
0.001 |
| Local Breast Recurrence |
15 |
44 |
||
| Local Chest Wall Recurrence |
6 |
14 |
||
| Regional Recurrence |
10 |
8 |
||
| Distant Recurrence |
140 |
167 |
||
| Distant recurrence (first or subsequent events) |
142 |
169 |
0.88 (0.7,1.1) |
0.246 |
| Contralateral Breast Cancer |
37 |
53 |
||
| Deaths Without Recurrence or Contralateral Breast Cancer |
135 |
116 |
14.4 First-Line Treatment of Advanced Breast Cancer
| Baseline Status | Letrozole N=458 |
Tamoxifen N=458 |
|---|---|---|
|
Stage of Disease
|
||
| IIIB |
6% |
7% |
| IV |
93% |
92% |
|
Receptor Status
|
||
| ER and PgR Positive |
38% |
41% |
| ER or PgR Positive |
26% |
26% |
| Both Unknown |
34% |
33% |
| ER- or PgR- / Other Unknown |
<1% |
0 |
|
Previous Antiestrogen Therapy
|
||
| Adjuvant |
19% |
18% |
| None |
81% |
82% |
|
Dominant Site of Disease
|
||
| Soft Tissue |
25% |
25% |
| Bone |
32% |
29% |
| Viscera |
43% |
46% |
| |
Letrozole
2.5 mg N=453 |
Tamoxifen
20 mg N=454 |
Hazard or Odds
Ratio (95% CI) P-Value (2-Sided) |
|
Median Time to Progression
|
9.4 months |
6 months |
0.72 (0.62, 0.83) P<0.0001 |
|
Objective Response Rate
|
|||
|
(CR + PR) |
145 (32%) |
95 (21%) |
1.77 (1.31, 2.39) P=0.0002 |
|
(CR) |
42 (9%) |
15 (3%) |
2.99 (1.63, 5.47) P=0.0004 |
|
Duration of Objective Response
|
|||
| Median |
18 months (N=145) |
16 months (N=95) |
|
|
Overall Survival
|
35 months (N=458) |
32 months (N=458) |
P=0.5136 |
Figure 2 Kaplan-Meier Estimates of Time to Progression (Tamoxifen Study)

| Variable | Letrozole 2.5 mg N=84 |
Tamoxifen 20 mg N=83 |
|---|---|---|
|
Median Time to Progression
(95% CI) |
8.9 months (6.2, 12.5) |
5.9 months (3.2, 6.2) |
| Hazard Ratio for TTP (95% CI) |
0.6 (0.43, 0.84) |
|
|
Objective Response Rate
|
||
| (CR + PR) |
22 (26%) |
7 (8%) |
| Odds Ratio for Response (95% CI) |
3.85 (1.5, 9.6) |
|
| Letrozole 2.5 mg | Tamoxifen 20 mg | |
|---|---|---|
|
Dominant Disease Site
|
||
|
Soft Tissue:
|
N=113 |
N=115 |
| Median TTP |
12.1 months |
6.4 months |
| Objective Response Rate |
50% |
34% |
|
Bone:
|
N=145 |
N=131 |
| Median TTP |
9.5 months |
6.3 months |
| Objective Response Rate |
23% |
15% |
|
Viscera:
|
N=195 |
N=208 |
| Median TTP |
8.3 months |
4.6 months |
| Objective Response Rate |
28% |
17% |
| Variable | Letrozole 2.5 mg | Tamoxifen 20 mg |
|---|---|---|
|
Receptor Positive
|
N=294 |
N=305 |
| Median Time to Progression (95% CI) |
9.4 months (8.9, 11.8) |
6 months (5.1, 8.5) |
| Hazard Ratio for TTP (95% CI) |
0.69 (0.58, 0.83) |
|
| Objective Response Rate (CR+PR) |
97 (33%) |
66 (22%) |
| Odds Ratio for Response (95% CI) |
1.78 (1.2, 2.6) |
|
|
Receptor Unknown
|
N=159 |
N=149 |
| Median Time to Progression (95% CI) |
9.2 months (6.1, 12.3) |
6 months (4.1, 6.4) |
| Hazard Ratio for TTP (95% CI) |
0.77 (0.6, 0.99) |
|
| Objective Response Rate (CR+PR) |
48 (30%) |
29 (20%) |
| Odds Ratio for Response (95% CI) |
1.79 (1.1, 3) |
|
Figure 3 Survival by Randomized Treatment Arm

Legend:
14.5 Second-Line Treatment of Advanced Breast Cancer
| Parameter | Megestrol Acetate Study | Aminoglutethimide Study |
|---|---|---|
|
No. of Participants
|
552 |
557 |
|
Receptor Status
|
||
| ER/PR Positive |
57% |
56% |
| ER/PR Unknown |
43% |
44% |
|
Previous Therapy
|
||
| Adjuvant Only |
33% |
38% |
| Therapeutic +/- Adj. |
66% |
62% |
|
Sites of Disease
|
||
| Soft Tissue |
56% |
50% |
| Bone |
50% |
55% |
| Viscera |
40% |
44% |
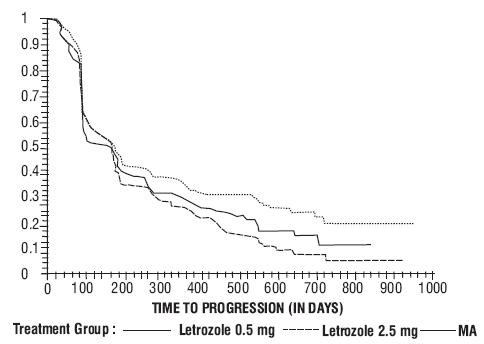
| Letrozole 0.5 mg N=188 |
Letrozole 2.5 mg N=174 |
Megestrol Acetate N=190 |
|
|---|---|---|---|
|
Objective Response (CR + PR)
|
22 (11.7%) |
41 (23.6%) |
31 (16.3%) |
|
Median Duration of Response
|
552 days |
(Not reached) |
561 days |
|
Median Time to Progression
|
154 days |
170 days |
168 days |
|
Median Survival
|
633 days |
730 days |
659 days |
|
Odds Ratio for Response
|
letrozole 2.5: letrozole 0.5=2.33 (95% CI: 1.32, 4.17); P=0.004  |
letrozole 2.5: megestrol=1.58 (95% CI: 0.94, 2.66); P=0.08  |
|
|
Relative Risk of Progression
|
letrozole 2.5: letrozole 0.5=0.81 (95% CI: 0.63, 1.03); P=0.09  |
letrozole 2.5: megestrol=0.77 (95% CI: 0.6, 0.98); P=0.03  |
|
Figure 4 Kaplan-Meier Estimates of Time to Progression (Megestrol Acetate Study)
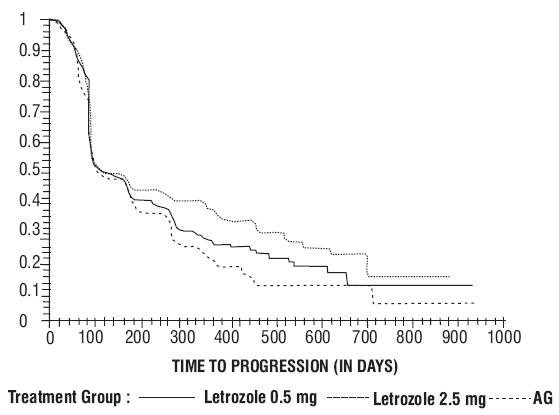
| |
Letrozole
0.5 mg N=193 |
Letrozole
2.5 mg N=185 |
Aminoglutethimide
N=179 |
|
Objective Response (CR + PR)
|
34 (17.6%) |
34 (18.4%) |
22 (12.3%) |
|
Median Duration of Response
|
619 days |
706 days |
450 days |
|
Median Time to Progression
|
103 days |
123 days |
112 days |
|
Median Survival
|
636 days |
792 days |
592 days |
|
Odds Ratio for
Response |
letrozole 2.5: letrozole 0.5=1.05 (95% CI: 0.62, 1.79); P=0.85 |
letrozole 2.5: aminoglutethimide=1.61 (95% CI: 0.9, 2.87); P=0.11  |
|
|
Relative Risk of Progression
|
letrozole 2.5: letrozole 0.5=0.86 (95% CI: 0.68, 1.11); P=0.25 |
letrozole 2.5: aminoglutethimide=0.74 (95% CI: 0.57, 0.94); P=0.02  |
|
Figure 5 Kaplan-Meier Estimates of Time to Progression (Aminoglutethimide Study)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Information for Patients
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Industries Ltd.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 62756-511-83
Letrozole Tablets USP
2.5 mg
Rx only
30 TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.

LETROZOLELETROZOLE TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!