LETROZOLE
Breckenridge Pharmaceutical, Inc.
Natco Pharma Ltd
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use letrozole tablets safely and effectively. See full prescribing information for letrozole tablets.Letrozole tablets USP, 2.5 mgInitial U.S. Approval: 1997 RECENT MAJOR CHANGESAdjuvant Treatment of Early Breast Cancer (1.1, 2.2) 04/2010INDICATIONS AND USAGE Adjuvant treatment of postmenopausal women with hormone receptor positive early breast cancer (1.1) Extended adjuvant treatment of postmenopausal women with early breast cancer who have received prior standard adjuvant tamoxifen therapy (1.2) First and second-line treatment of postmenopausal women with hormone receptor positive or unknown advanced breast cancer (1.3) DOSAGE AND ADMINISTRATION Recommended dose: 2.5 mg once daily (2.1) Patients with cirrhosis or severe hepatic impairment: 2.5 mg every other day (2.5, 5.3) DOSAGE FORMS AND STRENGTHSCONTRAINDICATIONSWARNINGS AND PRECAUTIONS Decreases in bone mineral density may occur. Consider bone mineral density monitoring (5.1) Increases in total cholesterol may occur. Consider cholesterol monitoring. (5.2) Fatigue, dizziness and somnolence may occur. Exercise caution when operating machinery (5.4) Side Effects To report SUSPECTED ADVERSE REACTIONS, contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 LETROZOLE INDICATIONS AND USAGE
- 2 LETROZOLE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 LETROZOLE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 LETROZOLE ADVERSE REACTIONS
- 6.1 Adjuvant Treatment of Early Breast Cancer
- 6.2 Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 24 Months
- 6.3 Updated Analysis, Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 60 Months
- 6.4 First-Line Treatment of Advanced Breast Cancer
- 6.5 Second- Line Treatment of Advanced Breast Cancer
- 6.6 First and Second-Line Treatment of Advanced Breast Cancer
- 6.7 Postmarketing Experience
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 LETROZOLE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 14.1 Updated Adjuvant Treatment of Early Breast Cancer
- 14.2 Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 24 Months
- 14.3 Updated Analyses of Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 60 Months
- 14.4 First-Line Treatment of Advanced Breast Cancer
- 14.5 Second-Line Treatment of Advanced Breast Cancer
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- Letrozole tablets 2.5 mg-Bottle of 30 tablets
- Letrozole tablets 2.5 mg-Bottle of 1000 tablets
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Adjuvant Treatment of Early Breast Cancer
Letrozole tablets are indicated for the adjuvant treatment of postmenopausal women with hormone receptor positive early breast cancer.
1.2 Extended Adjuvant Treatment of Early Breast Cancer
Letrozole tablets are indicated for the extended adjuvant treatment of early breast cancer in postmenopausal women, who have received 5 years of adjuvant tamoxifen therapy. The effectiveness of letrozole tablets in extended adjuvant treatment of early breast cancer is based on an analysis of disease-free survival in patients treated with letrozole tablets for a median of 60 months [see Clinical Studies (14.2, 14.3)].
1.3 First and Second-Line Treatment of Advanced Breast Cancer
[see Clinical Studies (14.4, 14.5)].
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The recommended dose of letrozole tablets is one 2.5 mg tablet administered once a day, without regard to meals.
2.2 Use in Adjuvant Treatment of Early Breast Cancer
[see Clinical Studies (14.1)].
2.3 Use in Extended Adjuvant Treatment of Early Breast Cancer
[see Clinical Studies (14.2 )].
2.4 Use in First and Second-Line Treatment of Advanced Breast Cancer
[see Clinical Studies (14.4, 14.5)]
2.5 Use in Hepatic Impairment
[see Warnings and Precautions (5.3)].
2.6 Use in Renal Impairment
[see Clinical Pharmacology (12.3)]
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
[see Use in Specific Populations (8.1)]
5 WARNINGS AND PRECAUTIONS
5.1 Bone Effects
Psee Adverse Reactions (6.1) [see Adverse Reactions (6.2)].
See Adverse Reactions (6.1) [see Adverse Reactions (6.3)].
5.2 Cholesterol
[see Adverse Reactions (6.2)].
5.3 Hepatic Impairment
[see Dosage and Administration (2.5)]
5.4 Fatigue and Dizziness
5.5 Laboratory Test Abnormalities
6 ADVERSE REACTIONS
- Bone effects [see Warnings and Precautions (5.1)]
- Increases in cholesterol [see Warnings and Precautions (5.2)]
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Adjuvant Treatment of Early Breast Cancer
Table 1: Patients with Adverse Reactions (CTC Grades 1-4, Irrespective of Relationship to Study Drug) in the Adjuvant Study – Monotherapy Arms Analysis (Median Follow-up 73 Months; Median Treatment 60 Months)
|
|
Grades 1-4
|
Grades 3-4
|
||||||
|
Adverse Reaction
|
Letrozole tablets
N=2448 n (%) |
tamoxifen
N=2447 n (%) |
Letrozole tablets
N=2448 n (%) |
tamoxifen
N=2447 n (%) |
||||
| Pts with any adverse event |
2310 |
(94.4) |
2214 |
(90.5) |
635 |
(25.9) |
604 |
(24.7) |
| Hypercholesterolemia |
1280 |
(52.3) |
700 |
(28.6) |
11 |
( 0.4) |
6 |
( 0.2) |
| Hot Flashes/Flushes |
821 |
(33.5) |
929 |
(38.0) |
0 |
- |
0 |
- |
| Arthralgia/Arthritis |
618 |
(25.2) |
501 |
(20.4) |
85 |
( 3.5) |
50 |
( 2.0) |
| Night Sweats |
357 |
(14.6) |
426 |
(17.4) |
0 |
- |
0 |
- |
| Bone Fractures2
|
338 |
(13.8) |
257 |
(10.5) |
- |
- |
- |
- |
| Weight Increase |
317 |
(12.9) |
378 |
(15.4) |
27 |
( 1.1) |
39 |
( 1.6) |
| Nausea |
283 |
(11.6) |
277 |
(11.3) |
6 |
( 0.2) |
9 |
( 0.4) |
| Bone Fractures1
|
247 |
(10.1) |
174 |
( 7.1) |
- |
- |
- |
- |
| Fatigue (Lethargy, Malaise, Asthenia) |
235 |
(9.6) |
250 |
(10.2) |
6 |
( 0.2) |
7 |
( 0.3) |
| Myalgia |
217 |
( 8.9) |
212 |
( 8.7) |
18 |
( 0.7) |
14 |
( 0.6) |
| Edema |
164 |
( 6.7) |
160 |
( 6.5) |
3 |
( 0.1) |
1 |
(<0.1) |
| Weight Decrease |
140 |
( 5.7) |
129 |
( 5.3) |
8 |
( 0.3) |
5 |
( 0.2) |
| Vaginal Bleeding |
128 |
( 5.2) |
320 |
(13.1) |
1 |
(<0.1) |
8 |
( 0.3) |
| Back Pain |
125 |
( 5.1) |
136 |
( 5.6) |
7 |
( 0.3) |
11 |
( 0.4) |
| Osteoporosis NOS |
124 |
( 5.1) |
66 |
( 2.7) |
10 |
( 0.4) |
5 |
( 0.2) |
| Bone pain |
123 |
( 5.0) |
109 |
( 4.5) |
6 |
( 0.2) |
4 |
( 0.2) |
| Depression |
119 |
( 4.9) |
114 |
( 4.7) |
16 |
( 0.7) |
14 |
( 0.6) |
| Vaginal Irritation |
111 |
( 4.5) |
77 |
( 3.1) |
2 |
(<0.1) |
2 |
(<0.1) |
| Headache |
105 |
( 4.3) |
94 |
( 3.8) |
9 |
( 0.4) |
5 |
( 0.2) |
| Pain in extremity |
103 |
( 4.2) |
79 |
( 3.2) |
6 |
( 0.2) |
4 |
( 0.2) |
| Osteopenia |
87 |
( 3.6) |
74 |
( 3.0) |
0 |
- |
2 |
(<0.1) |
| Dizziness/Light-Headedness |
84 |
( 3.4) |
84 |
( 3.4) |
1 |
(<0.1) |
6 |
(0.2) |
| Alopecia |
83 |
( 3.4) |
84 |
( 3.4) |
0 |
- |
0 |
- |
| Vomiting |
80 |
( 3.3) |
80 |
( 3.3) |
3 |
( 0.1) |
5 |
(0.2) |
| Cataract |
49 |
( 2.0) |
54 |
( 2.2) |
16 |
( 0.7) |
17 |
( 0.7) |
| Constipation |
49 |
( 2.0) |
71 |
( 2.9) |
3 |
( 0.1) |
1 |
(<0.1) |
| Breast pain |
37 |
( 1.5) |
43 |
( 1.8) |
1 |
(<0.1) |
0 |
- |
| Anorexia |
20 |
( 0.8) |
20 |
( 0.8) |
1 |
(<0.1) |
1 |
(<0.1) |
| Endometrial Hyperplasia/ Cancer2, 3
|
11/1909 |
( 0.6) |
70/1943 |
( 3.6) |
- |
- |
- |
- |
| Endometrial Proliferation Disorders |
10 |
(0.3) |
71 |
(1.8) |
0 |
- |
14 |
(0.6) |
| Endometrial Hyperplasia/ Cancer1, 3
|
6/1909 |
( 0.3) |
57/1943 |
(2.9) |
- |
- |
- |
- |
| Other Endometrial Disorders |
2 |
(<0.1) |
3 |
( 0.1) |
0 |
- |
0 |
- |
| Myocardial Infarction1
|
24 |
( 1.0) |
12 |
( 0.5) |
- |
- |
- |
- |
| Myocardial Infarction2
|
37 |
( 1.5) |
25 |
(1.0) |
- |
- |
- |
- |
| Myocardial Ischemia |
6 |
( 0.2) |
9 |
( 0.4) |
- |
- |
- |
- |
| Cerebrovascular Accident1
|
52 |
( 2.1) |
46 |
( 1.9) |
- |
- |
- |
- |
| Cerebrovascular Accident2
|
70 |
( 2.9) |
63 |
( 2.6) |
- |
- |
- |
- |
| Angina1
|
26 |
( 1.1) |
24 |
( 1.0) |
- |
- |
- |
- |
| Angina2
|
32 |
( 1.3) |
31 |
( 1.3) |
- |
- |
- |
- |
| Thromboembolic Event1
|
51 |
( 2.1) |
89 |
( 3.6) |
- |
- |
- |
- |
| Thromboembolic Event2
|
71 |
( 2.9) |
111 |
( 4.5) |
- |
- |
- |
- |
| Other Cardiovascular1
|
260 |
(10.6) |
256 |
(10.5) |
- |
- |
- |
- |
| Other Cardiovascular2
|
312 |
(12.7) |
337 |
(13.8) |
- |
- |
- |
- |
| Second Malignancies1
|
53 |
( 2.2) |
78 |
( 3.2) |
- |
- |
- |
- |
| Second Malignancies2
|
102 |
( 4.2) |
119 |
( 4.9) |
- |
- |
- |
- |
1 During study treatment, based on Safety Monotherapy population
2 Any time after randomization, including post treatment follow-up
3 Excluding women who had undergone hysterectomy before study entry
P
6.2 Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 24 Months
Table 2: Percentage of Patients with Adverse Reactions
| |
Number (%) of Patients with Grade 1-4 Adverse Reaction
|
Number (%) of Patients with Grade 3-4 Adverse Reaction
|
|||
| |
Letrozole tablets
N=2563 |
Placebo
N=2573 |
Letrozole tablets
N=2563 |
Placebo
N=2573 |
|
|
Any Adverse Reaction
|
2232 (87.1) |
2174 (84.5) |
419 (16.3) |
389 (15.1) |
|
|
Vascular Disorders
|
1375 (53.6) |
1230 (47.8) |
59 (2.3) |
74 (2.9) |
|
|
|
Flushing |
1273 (49.7) |
1114 (43.3) |
3 (0.1) |
0 - |
|
General Disorders
|
1154 (45) |
1090 (42.4) |
30 (1.2) |
28 (1.1) |
|
|
|
Asthenia |
862 (33.6) |
826 (32.1) |
16 (0.6) |
7 (0.3) |
|
|
Edema NOS |
471 (18.4) |
416 (16.2) |
4 (0.2) |
3 (0.1) |
|
Musculoskeletal Disorders
|
978 (38.2) |
836 (32.5) |
71 (2.8) |
50 (1.9) |
|
|
|
Arthralgia |
565 (22) |
465 (18.1) |
25 (1) |
20 (0.8) |
|
|
Arthritis NOS |
173 (6.7) |
124 (4.8) |
10 (0.4) |
5 (0.2) |
|
|
Myalgia |
171 (6.7) |
122 (4.7) |
8 (0.3) |
6 (0.2) |
|
|
Back Pain |
129 (5) |
112 (4.4) |
8 (0.3) |
7 (0.3) |
|
Nervous System Disorders
|
863 (33.7) |
819 (31.8) |
65 (2.5) |
58 (2.3) |
|
|
|
Headache |
516 (20.1) |
508 (19.7) |
18 (0.7) |
17 (0.7) |
|
|
Dizziness |
363 (14.2) |
342 (13.3) |
9 (0.4) |
6 (0.2) |
|
Skin Disorders
|
830 (32.4) |
787 (30.6) |
17 (0.7) |
16 (0.6) |
|
|
|
Sweating Increased |
619 (24.2) |
577 (22.4) |
1 (<0.1) |
0 - |
|
Gastrointestinal Disorders
|
725 (28.3) |
731 (28.4) |
43 (1.7) |
42 (1.6) |
|
|
|
Constipation |
290 (11.3) |
304 (11.8) |
6 (0.2) |
2 (<0.1) |
|
|
Nausea |
221 (8.6) |
212 (8.2) |
3 (0.1) |
10 (0.4) |
|
|
Diarrhea NOS |
128 (5) |
143 (5.6) |
12 (0.5) |
8 (0.3) |
|
Metabolic Disorders
|
551 (21.5) |
537 (20.9) |
24 (0.9) |
32 (1.2) |
|
|
|
Hypercholesterolemia |
401 (15.6) |
398 (15.5) |
2 (<0.1) |
5 (0.2) |
|
Reproductive Disorders
|
303 (11.8) |
357 (13.9) |
9 (0.4) |
8 (0.3) |
|
|
|
Vaginal Hemorrhage |
123 (4.8) |
171 (6.6) |
2 (<0.1) |
5 (0.2) |
|
|
Vulvovaginal Dryness |
137 (5.3) |
127 (4.9) |
0 - |
0 - |
|
Psychiatric Disorders
|
320 (12.5) |
276 (10.7) |
21 (0.8) |
16 (0.6) |
|
|
|
Insomnia |
149 (5.8) |
120 (4.7) |
2 (<0.1) |
2 (<0.1) |
|
Respiratory Disorders
|
279 (10.9) |
260 (10.1) |
30 (1.2) |
28 (1.1) |
|
|
|
Dyspnea |
140 (5.5) |
137 (5.3) |
21 (0.8) |
18 (0.7) |
|
Investigations
|
184 (7.2) |
147 (5.7) |
13 (0.5) |
13 (0.5) |
|
|
Infections and Infestations
|
166 (6.5) |
163 (6.3) |
40 (1.6) |
33 (1.3) |
|
|
Renal Disorders
|
130 (5.1) |
100 (3.9) |
12 (0.5) |
6 (0.2) |
|
Bone Sub-study: [see Warnings and Precautions (5.1)].
Lipid Sub-study: [see Warnings and Precautions (5.2)].
6.3 Updated Analysis, Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 60 Months
see Adverse Reactions ( 6.2 )
[see Warnings and Precautions (5.2)]
6.4 First-Line Treatment of Advanced Breast Cancer
Table 3: Percentage (%) of Patients with Adverse Reactions
|
Adverse
Reaction
|
Letrozole tablets
2.5 mg (N=455) % |
tamoxifen
20 mg (N=455) % |
|
|
General Disorders
|
|
|
|
| |
Fatigue |
13 |
13 |
| |
Chest Pain |
8 |
9 |
| |
Edema Peripheral |
5 |
6 |
| |
Pain NOS |
5 |
7 |
| |
Weakness |
6 |
4 |
|
Investigations
|
|
|
|
| |
Weight Decreased |
7 |
5 |
|
Vascular Disorders
|
|
|
|
| |
Hot Flushes |
19 |
16 |
| |
Hypertension |
8 |
4 |
|
Gastrointestinal Disorders
|
|
|
|
| |
Nausea |
17 |
17 |
| |
Constipation |
10 |
11 |
| |
Diarrhea |
8 |
4 |
| |
Vomiting |
7 |
8 |
|
Infections/Infestations
|
|
|
|
| |
Influenza |
6 |
4 |
| |
Urinary Tract Infection NOS |
6 |
3 |
|
Injury, Poisoning and Procedural Complications
|
|
|
|
| |
Post-Mastectomy Lymphedema |
7 |
7 |
|
Metabolism and Nutrition Disorders
|
|
|
|
| |
Anorexia |
4 |
6 |
|
Musculoskeletal and Connective Tissue Disorders
|
|
|
|
| |
Bone Pain |
22 |
21 |
| |
Back Pain |
18 |
19 |
| |
Arthralgia |
16 |
15 |
| |
Pain in Limb |
10 |
8 |
|
Nervous System Disorders
|
|
|
|
| |
Headache NOS |
8 |
7 |
|
Psychiatric Disorders
|
|
|
|
| |
Insomnia |
7 |
4 |
|
Reproductive System and Breast Disorders
|
|
|
|
| |
Breast Pain |
7 |
7 |
|
Respiratory, Thoracic and Mediastinal Disorders
|
|
|
|
| |
Dyspnea |
18 |
17 |
| |
Cough |
13 |
13 |
| |
Chest Wall Pain |
6 |
6 |
6.5 Second- Line Treatment of Advanced Breast Cancer
Table 4: Percentage (%) of Patients with Adverse Reactions
|
Adverse
Reaction
|
Pooled
letrozole tablets 2.5 mg (N=359) % |
Pooled
letrozole tablets 0.5 mg (N=380) % |
megestrol acetate 160 mg (N=189) % |
aminoglutethimide 500 mg (N=178) % |
|
|
Body as a Whole
|
|
|
|
|
|
| |
Fatigue |
8 |
6 |
11 |
3 |
| |
Chest Pain |
6 |
3 |
7 |
3 |
| |
Peripheral Edema1
|
5 |
5 |
8 |
3 |
| |
Asthenia |
4 |
5 |
4 |
5 |
| |
Weight Increase |
2 |
2 |
9 |
3 |
|
Cardiovascular
|
|
|
|
|
|
| |
Hypertension |
5 |
7 |
5 |
6 |
|
Digestive System
|
|
|
|
|
|
| |
Nausea |
13 |
15 |
9 |
14 |
| |
Vomiting |
7 |
7 |
5 |
9 |
| |
Constipation |
6 |
7 |
9 |
7 |
| |
Diarrhea |
6 |
5 |
3 |
4 |
| |
Pain-Abdominal |
6 |
5 |
9 |
8 |
| |
Anorexia |
5 |
3 |
5 |
5 |
| |
Dyspepsia |
3 |
4 |
6 |
5 |
|
Infections/Infestations
|
|
|
|
|
|
| |
Viral Infection |
6 |
5 |
6 |
3 |
|
Lab Abnormality
|
|
|
|
|
|
| |
Hypercholesterolemia |
3 |
3 |
0 |
6 |
|
Musculoskeletal System
|
|
|
|
|
|
| |
Musculoskeletal2
|
21 |
22 |
30 |
14 |
| |
Arthralgia |
8 |
8 |
8 |
3 |
|
Nervous System
|
|
|
|
|
|
| |
Headache |
9 |
12 |
9 |
7 |
| |
Somnolence |
3 |
2 |
2 |
9 |
| |
Dizziness |
3 |
5 |
7 |
3 |
|
Respiratory System
|
|
|
|
|
|
| |
Dyspnea |
7 |
9 |
16 |
5 |
| |
Coughing |
6 |
5 |
7 |
5 |
|
Skin and Appendages
|
|
|
|
|
|
| |
Hot Flushes |
6 |
5 |
4 |
3 |
| |
Rash3
|
5 |
4 |
3 |
12 |
| |
Pruritus |
1 |
2 |
5 |
3 |
1
2 Includes musculoskeletal pain, skeletal pain, back pain, arm pain, leg pain
3 Includes rash, erythematous rash, maculopapular rash, psoriasiform rash, vesicular rash
6.6 First and Second-Line Treatment of Advanced Breast Cancer
6.7 Postmarketing Experience
7 DRUG INTERACTIONS
Tamoxifen
Cimetidine
Warfarin
Other anticancer agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category X [see Contraindications (4)].
2
222[see Nonclinical Toxicology (13.2)].
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
22
11 DESCRIPTION
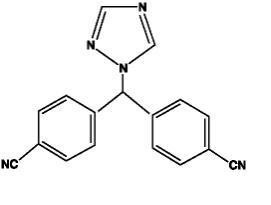
17115
Inactive Ingredients:
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
Absorption and Distribution: Letrozole is rapidly and completely absorbed from the gastrointestinal tract and absorption is not affected by food. It is metabolized slowly to an inactive metabolite whose glucuronide conjugate is excreted renally, representing the major clearance pathway. About 90% of radiolabeled letrozole is recovered in urine. Letrozole’s terminal elimination half-life is about 2 days and steady-state plasma concentration after daily 2.5 mg dosing is reached in 2-6 weeks. Plasma concentrations at steady state are 1.5 to 2 times higher than predicted from the concentrations measured after a single dose, indicating a slight non-linearity in the pharmacokinetics of letrozole upon daily administration of 2.5 mg. These steady-state levels are maintained over extended periods, however, and continuous accumulation of letrozole does not occur. Letrozole is weakly protein bound and has a large volume of distribution (approximately 1.9 L/kg).
Metabolism and Excretion: Metabolism to a pharmacologically-inactive carbinol metabolite (4,4'-methanol-bisbenzonitrile) and renal excretion of the glucuronide conjugate of this metabolite is the major pathway of letrozole clearance. Of the radiolabel recovered in urine, at least 75% was the glucuronide of the carbinol metabolite, about 9% was two unidentified metabolites, and 6% was unchanged letrozole.
In human microsomes with specific CYP isozyme activity, CYP3A4 metabolized letrozole to the carbinol metabolite while CYP2A6 formed both this metabolite and its ketone analog. In human liver microsomes, letrozole strongly inhibited CYP2A6 and moderately inhibited CYP2C19.
Pediatric, Geriatric and Race: In the study populations (adults ranging in age from 35 to >80 years), no change in pharmacokinetic parameters was observed with increasing age. Differences in letrozole pharmacokinetics between adult and pediatric populations have not been studied. Differences in letrozole pharmacokinetics due to race have not been studied.
Renal Impairment: In a study of volunteers with varying renal function (24-hour creatinine clearance: 9-116 mL/min), no effect of renal function on the pharmacokinetics of single doses of 2.5 mg of letrozole tablets was found. In addition, in a study of 347 patients with advanced breast cancer, about half of whom received 2.5 mg letrozole tablets and half 0.5 mg letrozole tablets, renal impairment (calculated creatinine clearance: 20-50 mL/min) did not affect steady-state plasma letrozole concentrations.
Hepatic Impairment:
[see Dosage and Administration (2.5)]
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis , Impairment Of Fertility
20-12hr0-24hr 20-24hr
in vitroin vitroin vivo
2
Letrozole administered to young (postnatal day 7) rats for 12 weeks duration at 0.003, 0.03, 0.3 mg/kg/day by oral gavage, resulted in adverse skeletal/growth effects (bone maturation, bone mineral density) and neuroendocrine and reproductive developmental perturbations of the hypothalamic-pituitary axis at exposures less than exposure anticipated at the clinical dose of 2.5 mg/day. Decreased fertility was accompanied by hypertrophy of the hypophysis and testicular changes that included degeneration of the seminiferous tubular epithelium and atrophy of the female reproductive tract. Young rats in this study were allowed to recover following discontinuation of letrozole treatment for 42 days. Histopathological changes were not reversible at clinically relevant exposures.
13.2 Animal Pharmacology and/or Toxicology
Reproductive Toxicology: 22
2
14 CLINICAL STUDIES
14.1 Updated Adjuvant Treatment of Early Breast Cancer
The study in the adjuvant setting, BIG 1-98 was designed to answer two primary questions: whether letrozole tablets for 5 years were superior to tamoxifen for 5 years (Primary Core Analysis) and whether switching endocrine treatments at 2 years was superior to continuing the same agent for a total of 5 years (Sequential Treatments Analysis). Selected baseline characteristics for the study population are shown in Table 5.
The primary endpoint of this trial was disease-free survival (DFS) (i.e., interval between randomization and earliest occurrence of a local, regional, or distant recurrence, or invasive contralateral breast cancer, or death from any cause). The secondary endpoints were overall survival (OS), systemic disease-free survival (SDFS), invasive contralateral breast cancer, time to breast cancer recurrence (TBR) and time to distant metastasis (TDM).
The Primary Core Analysis (PCA) included all patients and all follow-up in the monotherapy arms in both randomization options, but follow-up in the two sequential treatments arms was truncated 30 days after switching treatments. The PCA was conducted at a median treatment duration of 24 months and a median follow-up of 26 months. Letrozole tablets were superior to tamoxifen in all endpoints except overall survival and contralateral breast cancer [e.g., DFS: hazard ratio, HR 0.79; 95% CI (0.68, 0.92); P=0.002; SDFS: HR 0.83; 95% CI (0.70, 0.97); TDM: HR 0.73; 95% CI (0.60, 0.88); OS: HR 0.86; 95% CI (0.70, 1.06).
In 2005, based on recommendations by the independent Data Monitoring Committee, the tamoxifen arms were unblinded and patients were allowed to complete initial adjuvant therapy with letrozole tablets (if they had received tamoxifen for at least 2 years) or to start extended adjuvant treatment with letrozole tablets (if they had received tamoxifen for at least 4.5 years) if they remained alive and disease-free. In total, 632 patients crossed to letrozole tablets or another aromatase inhibitor. Approximately 70% (448) of these 632 patients crossed to letrozole tablets to complete initial adjuvant therapy and most of these crossed in years 3 to 4. All of these patients were in Option 1. A total of 184 patients started extended adjuvant therapy with letrozole tablets (172 patients) or with another aromatase inhibitor (12 patients). To explore the impact of this selective crossover, results from analyses censoring follow-up at the date of the selective crossover (in the tamoxifen arm) are presented for the Monotherapy Arms Analysis (MAA).
The PCA allowed the results of letrozole tablets for 5 years compared with tamoxifen for 5 years to be reported in 2005 after a median follow-up of only 26 months. The design of the PCA is not optimal to evaluate the effect of letrozole tablets after a longer time (because follow-up was truncated in two arms at around 25 months). The Monotherapy Arms Analysis (ignoring the two sequential treatment arms) provided follow-up equally as long in each treatment and did not over-emphasize early recurrences as the PCA did. The MAA thus provides the clinically appropriate updated efficacy results in answer to the first primary question, despite the confounding of the tamoxifen reference arm by the selective crossover to letrozole tablets. The updated results for the MAA are summarized in Table 6. Median follow-up for this analysis is 73 months.
The Sequential Treatments Analysis (STA) addresses the second primary question of the study. The primary analysis for the Sequential Treatments Analysis (STA) was from switch (or equivalent time-point in monotherapy arms) + 30 days (STA-S) with a two-sided test applied to each pair-wise comparison at the 2.5% level. Additional analyses were conducted from randomization (STA-R) but these comparisons (added in light of changing medical practice) were under-powered for efficacy.
Table 5: Adjuvant Study - Patient and Disease Characteristics (ITT Population)
| |
Primary Core Analysis
(PCA) |
Monotherapy Arms Analysis (MAA)
|
|||
|
Characteristic |
Letrozole tablets
N=4003 n (%) |
tamoxifen
N=4007 n (%) |
Letrozole tablets
N=2463 n (%) |
tamoxifen
N=2459 n (%) |
|
| Age (median, years) |
61 |
61 |
61 |
61 |
|
| Age range (years) |
38-89 |
39-90 |
38-88 |
39-90 |
|
| Hormone receptor status (%) |
|
|
|
|
|
| |
ER+ and/or PgR+ |
99.7 |
99.7 |
99.7 |
99.7 |
| |
Both unknown |
0.3 |
0.3 |
0.3 |
0.3 |
| Nodal status (%) |
|
|
|
|
|
| |
Node negative |
52 |
52 |
50 |
52 |
| |
Node positive |
41 |
41 |
43 |
41 |
| |
Nodal status unknown |
7 |
7 |
7 |
7 |
| Prior adjuvant chemotherapy (%) |
24 |
24 |
24 |
24 |
|
Table 6: Updated Adjuvant Study Results - Monotherapy Arms Analysis
(Median Follow-up 73 Months)
| |
|
Letrozole tablets N=2463 |
tamoxifen
N=2459 |
Hazard ratio
|
|
||||
| |
|
Events
(%) |
5-year rate
|
Events
(%) |
5-year rate
|
(95% CI)
|
P
|
||
| Disease-free survival1
|
ITT |
445 (18.1) |
87.4 |
500 (20.3) |
84.7 |
0.87 (0.76, 0.99) |
0.03 |
||
| |
Censor |
445 |
87.4 |
483 |
84.2 |
0.84 (0.73, 0.95) |
|
||
| |
0 positive nodes |
ITT |
165 |
92.2 |
189 |
90.3 |
0.88 (0.72, 1.09) |
|
|
| |
1-3 positive nodes |
ITT |
151 |
85.6 |
163 |
83.0 |
0.85 (0.68, 1.06) |
|
|
| |
>=4 positive nodes |
ITT |
123 |
71.2 |
142 |
62.6 |
0.81 (0.64, 1.03) |
|
|
| |
Adjuvant chemotherapy |
ITT |
119 |
86.4 |
150 |
80.6 |
0.77 (0.60, 0.98) |
|
|
| |
No chemotherapy |
ITT |
326 |
87.8 |
350 |
86.1 |
0.91 (0.78, 1.06) |
|
|
| Systemic DFS2
|
ITT |
401 |
88.5 |
446 |
86.6 |
0.88 (0.77, 1.01) |
|
||
| Time to distant metastasis3
|
ITT |
257 |
92.4 |
298 |
90.1 |
0.85 (0.72, 1.00) |
|
||
| |
Adjuvant chemotherapy |
ITT |
84 |
- |
109 |
- |
0.75 (0.56, 1.00) |
|
|
| |
No chemotherapy |
ITT |
173 |
- |
189 |
- |
0.90 (0.73, 1.11) |
|
|
| Distant DFS4
|
ITT |
385 |
89.0 |
432 |
87.1 |
0.87 (0.76, 1.00) |
|
||
| Contralateral breast cancer |
ITT |
34 |
99.2 |
44 |
98.6 |
0.76 (0.49, 1.19) |
|
||
| Overall survival |
ITT |
303 |
91.8 |
343 |
90.9 |
0.87 (0.75, 1.02) |
|
||
| |
Censor |
303 |
91.8 |
338 |
90.1 |
0.82 (0.70, 0.96) |
|
||
| |
0 positive nodes |
ITT |
107 |
95.2 |
121 |
94.8 |
0.90 (0.69, 1.16) |
|
|
| |
1-3 positive nodes |
ITT |
99 |
90.8 |
114 |
90.6 |
0.81 (0.62, 1.06) |
|
|
| |
>=4 positive nodes |
ITT |
92 |
80.2 |
104 |
73.6 |
0.86 (0.65, 1.14) |
|
|
| |
Adjuvant chemotherapy |
ITT |
76 |
91.5 |
96 |
88.4 |
0.79 (0.58, 1.06) |
|
|
| |
No chemotherapy |
ITT |
227 |
91.9 |
247 |
91.8 |
0.91 (0.76, 1.08) |
|
|
| Definition of: 1 Disease-free survival: Interval from randomization to earliest event of invasive loco-regional recurrence, distant metastasis, invasive contralateral breast cancer, or death without a prior event 2 Systemic disease-free survival: Interval from randomization to invasive regional recurrence, distant metastasis, or death without a prior cancer event 3 Time to distant metastasis: Interval from randomization to distant metastasis 4 Distant disease-free survival: Interval from randomization to earlier event of relapse in a distant site or death from any cause ITT analysis ignores selective crossover in tamoxifen arms Censored analysis censors follow-up at the date of selective crossover in 632 patients who crossed to letrozole tablets or another aromatase inhibitor after the tamoxifen arms were unblinded in 2005 |
|||||||||
Figure 1 shows the Kaplan-Meier curves for Disease-Free Survival Monotherapy Analysis
Figure 1 Disease-Free Survival (Median follow-up 73 months, ITT Approach)
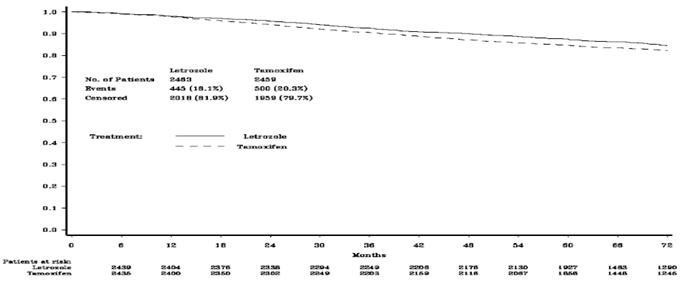
The medians of overall survival for both arms were not reached for the Monotherapy Arms Analysis (MAA). There was no statistically significant difference in overall survival. The hazard ratio for survival in the letrozole tablets arm compared to the tamoxifen arm was 0.87, with 95% CI (0.75, 1.02) (see Table 6).
There were no significant differences in DFS, OS, SDFS, and Distant DFS from switch in the Sequential Treatments Analysis with respect to either monotherapy (e.g., [Tamoxifen 2 years followed by] letrozole tablets 3 years versus tamoxifen beyond 2 years, DFS HR 0.89; 97.5% CI 0.68, 1.15 and [letrozole tablets 2 years followed by] tamoxifen 3 years versus letrozole tablets beyond 2 years, DFS HR 0.93; 97.5% CI 0.71, 1.22).
There were no significant differences in DFS, OS, SDFS, and Distant DFS from randomization in the Sequential Treatments Analyses.
14.2 Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 24 Months
Table 7: Selected Study Population Demographics (Modified ITT Population)
|
Baseline Status
|
Letrozole tablets
N=2582 |
Placebo
N=2586 |
|
|
Hormone Receptor Status (%)
|
|
|
|
| |
ER+ and/or PgR+ |
98 |
98 |
| |
Both Unknown |
2 |
2 |
|
Nodal Status (%)
|
|
|
|
| |
Node Negative |
50 |
50 |
| |
Node Positive |
46 |
46 |
| |
Nodal Status Unknown |
4 |
4 |
|
Chemotherapy
|
46 |
46 |
|
Table 8 shows the study results. Disease-free survival was measured as the time from randomization to the earliest event of loco-regional or distant recurrence of the primary disease or development of contralateral breast cancer or death. DFS by hormone receptor status, nodal status and adjuvant chemotherapy were similar to the overall results. Data were premature for an analysis of survival.
Table 8: Extended Adjuvant Study Results
|
|
Letrozole tablets N = 2582 |
Placebo N = 2586 |
Hazard Ratio (95% CI) |
P-Value
|
|
|
Disease Free Survival (DFS)1 Events
|
122 (4.7%) |
193 (7.5%) |
0.62 (0.49, 0.78)2
|
0.00003 |
|
| |
Local Breast Recurrence |
9 |
22 |
|
|
| |
Local Chest Wall Recurrence |
2 |
8 |
|
|
| |
Regional Recurrence |
7 |
4 |
|
|
| |
Distant Recurrence |
55 |
92 |
0.61 (0.44, 0.84) |
0.003 |
| |
Contralateral Breast Cancer |
19 |
29 |
|
|
| |
Deaths Without Recurrence or Contralateral Breast Cancer |
30 |
38 |
|
|
| CI = confidence interval for hazard ratio. Hazard ratio of less than 1.0 indicates difference in favor of letrozole tablets (lesser risk of recurrence); hazard ratio greater than 1.0 indicates difference in favor of placebo (higher risk of recurrence with letrozole tablets). 1 First event of loco-regional recurrence, distant relapse, contralateral breast cancer or death from any cause 2 Analysis stratified by receptor status, nodal status and prior adjuvant chemotherapy (stratification factors as at randomization). P-value based on stratified logrank test. |
|||||
14.3 Updated Analyses of Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 60 Months
Table 9: Update of Extended Adjuvant Study Results
| |
Letrozole tablets
N = 2582 (%) |
Placebo N = 2586 (%) |
Hazard Ratio1
(95% CI) |
P-Value2
|
|
|
Disease Free Survival (DFS) events3
|
344 (13.3) |
402 (15.5) |
0.89 (0.77, 1.03) |
0.12 |
|
|
Breast cancer recurrence
(Protocol definition of DFS events4) |
209 |
286 |
0.75 (0.63, 0.89) |
0.001 |
|
| |
Local Breast Recurrence |
15 |
44 |
|
|
| |
Local Chest Wall Recurrence |
6 |
14 |
|
|
| |
Regional Recurrence |
10 |
8 |
|
|
| |
Distant Recurrence |
140 |
167 |
|
|
| |
Distant recurrence (first or subsequent events) Contralateral Breast Cancer |
142 37 |
169 53 |
0.88 (0.70, 1.10) |
0.246 |
| |
Deaths Without Recurrence or Contralateral Breast Cancer |
135 |
116 |
|
|
|
1 Adjusted by receptor status, nodal status and prior chemotherapy 2 Stratified logrank test, stratified by receptor status, nodal status and prior chemotherapy 3 DFS events defined as earliest of loco-regional recurrence, distant metastasis, contralateral breast cancer or death from any cause, and ignoring switches to letrozole tablets in 60% of the placebo arm. 4 Protocol definition does not include deaths from any cause |
|||||
Updated analyses were conducted at a median follow-up of 62 months. In the letrozole tablets arm, 71% of the patients were treated for a least 3 years and 58% of patients completed at least 4.5 years of extended adjuvant treatment. After the unblinding of the study at a median follow-up of 28 months, approximately 60% of the selected patients in the placebo arm opted to switch to letrozole tablets.
P
14.4 First-Line Treatment of Advanced Breast Cancer
Table 10: Selected Study Population Demographics
|
Baseline Status
|
Letrozole tablets
N=458 |
tamoxifen
N=458 |
|
|
Stage of Disease
|
|
|
|
|
|
IIIB |
6% |
7% |
|
|
IV |
93% |
92% |
|
Receptor Status
|
|
|
|
|
|
ER and PgR Positive |
38% |
41% |
|
|
ER or PgR Positive |
26% |
26% |
|
|
Both Unknown |
34% |
33% |
|
|
ER- or PgR-/Other Unknown |
<1% |
0 |
|
Previous Antiestrogen Therapy
|
|
|
|
|
|
Adjuvant |
19% |
18% |
|
|
None |
81% |
82% |
|
Dominant Site of Disease
|
|
|
|
|
|
Soft Tissue |
25% |
25% |
|
|
Bone |
32% |
29% |
|
|
Viscera |
43% |
46% |
P
Table 11: Results of First-Line Treatment of Advanced Breast Cancer
|
|
Letrozole tablets
2.5 mg N=453 |
tamoxifen
20 mg N=454 |
Hazard or Odds
Ratio (95% CI) P-Value (2-Sided) |
|
|
Median Time to Progression
|
9.4 months |
6.0 months |
0.72 (0.62, 0.83)1
|
|
|
|
|
|
|
P<0.0001 |
|
Objective Response Rate
|
|
|
|
|
|
|
(CR + PR) |
145 (32%) |
95 (21%) |
1.77 (1.31, 2.39)2
|
|
|
|
|
|
P=0.0002 |
|
|
(CR) |
42 (9%) |
15 (3%) |
2.99 (1.63, 5.47)2
|
|
|
|
|
|
P=0.0004 |
|
Duration of Objective Response
|
|
|
|
|
|
|
Median |
18 months |
16 months |
|
|
|
|
(N=145) |
(N=95) |
|
|
Overall Survival
|
35 months |
32 months |
|
|
|
|
|
(N=458) |
(N=458) |
P=0.51363
|
1
2
3
Figure 2 Kaplan-Meier Estimates of Time to Progression (Tamoxifen Study)
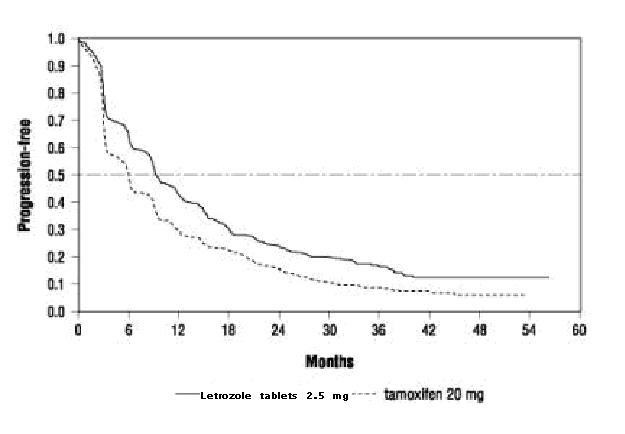
Table 12: Efficacy in Patients Who Received Prior Antiestrogen Therapy
|
Variable
|
Letrozole tablets
2.5 mg N=84 |
tamoxifen
20 mg N=83 |
|
|
Median Time to Progression (95% CI) |
8.9 months (6.2, 12.5) |
5.9 months (3.2, 6.2) |
|
|
|
Hazard Ratio for TTP (95% CI) |
|
0.60 (0.43, 0.84) |
|
Objective Response Rate
|
|
|
|
|
|
(CR + PR) |
22 (26%) |
7 (8%) |
|
|
Odds Ratio for Response (95% CI) |
|
3.85 (1.50, 9.60) |
Table 13: Efficacy by Disease Site
|
|
Letrozole tablets
2.5 mg |
tamoxifen
20 mg |
|
|
Dominant Disease Site
|
|
|
|
|
Soft Tissue:
|
N=113 |
N=115 |
|
|
|
Median TTP |
12.1 months |
6.4 months |
|
|
Objective Response Rate |
50% |
34% |
|
Bone:
|
N=145 |
N=131 |
|
|
|
Median TTP |
9.5 months |
6.3 months |
|
|
Objective Response Rate |
23% |
15% |
|
Viscera:
|
N=195 |
N=208 |
|
|
|
Median TTP |
8.3 months |
4.6 months |
|
|
Objective Response Rate |
28% |
17% |
Table 14: Efficacy by Receptor Status
|
Variable
|
Letrozole tablets
2.5 mg |
tamoxifen
20 mg |
|
Receptor Positive
|
N=294 |
N=305 |
| Median Time to Progression (95% CI) |
9.4 months (8.9, 11.8) |
6.0 months (5.1, 8.5) |
| Hazard Ratio for TTP (95% CI) |
0.69 (0.58, 0.83) |
|
| Objective Response Rate (CR+PR) |
97 (33%) |
66 (22%) |
| Odds Ratio for Response (95% CI) |
1.78 (1.20, 2.60) |
|
|
Receptor Unknown
|
N=159 |
N=149 |
| Median Time to Progression (95% CI) |
9.2 months (6.1, 12.3) |
6.0 months (4.1, 6.4) |
| Hazard Ratio for TTP (95% CI) |
0.77 (0.60, 0.99) |
|
| Objective Response Rate (CR+PR) |
48 (30%) |
29 (20%) |
| Odds Ratio for Response (95% CI) |
1.79 (1.10, 3.00) |
|
Figure 3 Survival by Randomized Treatment Arm
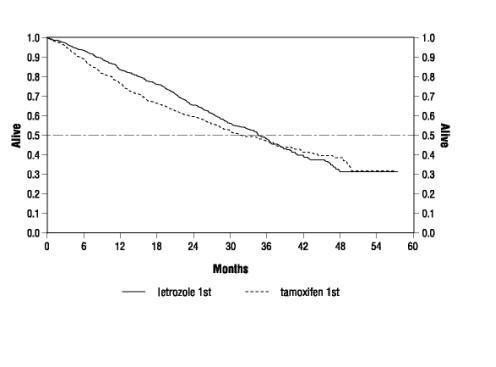
Legend:
P
P
14.5 Second-Line Treatment of Advanced Breast Cancer
Letrozole tablets was initially studied at doses of 0.1 mg to 5.0 mg daily in six non-comparative Phase I/II trials in 181 postmenopausal estrogen/progesterone receptor positive or unknown advanced breast cancer patients previously treated with at least antiestrogen therapy. Patients had received other hormonal therapies and also may have received cytotoxic therapy. Eight (20%) of forty patients treated with letrozole tablets 2.5 mg daily in Phase I/II trials achieved an objective tumor response (complete or partial response).
Two large randomized, controlled, multinational (predominantly European) trials were conducted in patients with advanced breast cancer who had progressed despite antiestrogen therapy. Patients were randomized to letrozole tablets 0.5 mg daily, letrozole tablets 2.5 mg daily, or a comparator (megestrol acetate 160 mg daily in one study; and aminoglutethimide 250 mg b.i.d. with corticosteroid supplementation in the other study). In each study over 60% of the patients had received therapeutic antiestrogens, and about one-fifth of these patients had had an objective response. The megestrol acetate controlled study was double-blind; the other study was open label. Selected baseline characteristics for each study are shown in Table 15.
Table 15: Selected Study Population Demographics
|
Parameter
|
megestrol acetate
study |
aminoglutethimide
study |
|
No. of Participants
|
552 |
557 |
|
Receptor Status
|
|
|
| ER/PR Positive |
57% |
56% |
| ER/PR Unknown |
43% |
44% |
|
Previous Therapy
|
|
|
| Adjuvant Only |
33% |
38% |
| Therapeutic +/- Adj. |
66% |
62% |
|
Sites of Disease
|
|
|
| Soft Tissue |
56% |
50% |
| Bone |
50% |
55% |
| Viscera |
40% |
44% |
Confirmed objective tumor response (complete response plus partial response) was the primary endpoint of the trials. Responses were measured according to the Union Internationale Contre le Cancer (UICC) criteria and verified by independent, blinded review. All responses were confirmed by a second evaluation 4-12 weeks after the documentation of the initial response.
Table 16 shows the results for the first trial, with a minimum follow-up of 15 months, that compared letrozole tablets 0.5 mg, letrozole tablets 2.5 mg, and megestrol acetate 160 mg daily. (All analyses are unadjusted.)
Table 16: Megestrol Acetate Study Results
| |
Letrozole tablets
0.5 mg N=188 |
Letrozole tablets
2.5 mg N=174 |
megestrol
acetate N=190 |
|
Objective Response (CR + PR)
|
22 (11.7%) |
41 (23.6%) |
31 (16.3%) |
|
Median Duration of Response
|
552 days |
(Not reached) |
561 days |
|
Median Time to Progression
|
154 days |
170 days |
168 days |
|
Median Survival
|
633 days |
730 days |
659 days |
|
Odds Ratio for Response
|
Letrozole tablets 2.5: Letrozole tablets 0.5=2.33 (95% CI: 1.32, 4.17); P=0.004* |
Letrozole tablets 2.5: megestrol=1.58 (95% CI: 0.94, 2.66); P=0.08* |
|
|
Relative Risk of Progression
|
Letrozole tablets 2.5: Letrozole tablets 0.5=0.81 (95% CI: 0.63, 1.03); P=0.09* |
Letrozole tablets 2.5: megestrol=0.77 (95% CI: 0.60, 0.98); P=0.03* |
|
Figure 4 Kaplan-Meier Estimates of Time to Progression (Megestrol Acetate Study)
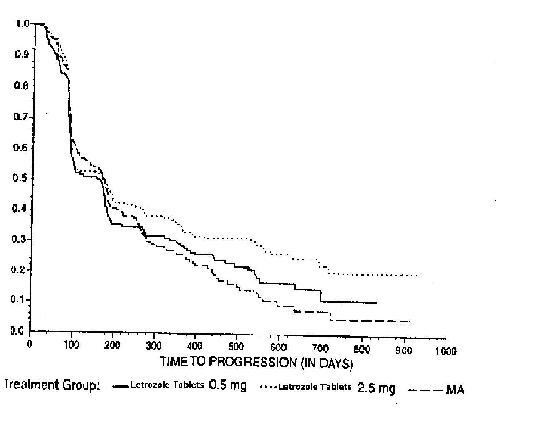
The results for the study comparing letrozole tablets to aminoglutethimide, with a minimum follow-up of 9 months, are shown in Table 17. (Unadjusted analyses are used.)
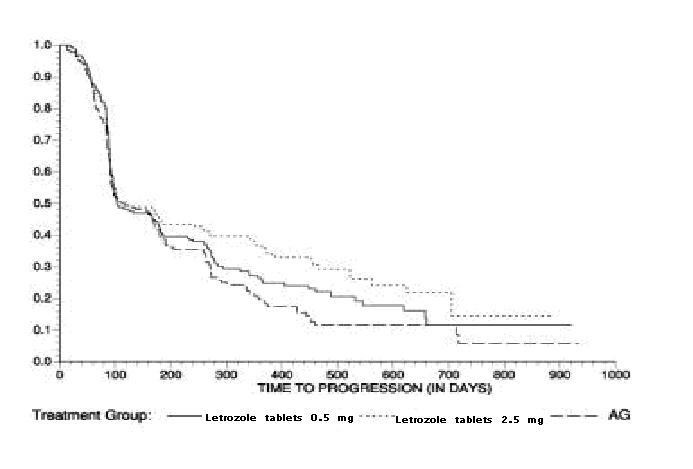
Table 17: Aminoglutethimide Study Results
| |
Letrozole tablets 0.5 mg N=193 |
Letrozole tablets
2.5 mg N=185 |
aminoglutethimide
N=179 |
|
Objective Response (CR + PR)
|
34 (17.6%) |
34 (18.4%) |
22 (12.3%) |
|
Median Duration of Response
|
619 days |
706 days |
450 days |
|
Median Time to Progression
|
103 days |
123 days |
112 days |
|
Median Survival
|
636 days |
792 days |
592 days |
|
Odds Ratio for Response
|
Letrozole tablets 2.5: Letrozole tablets 0.5=1.05 (95% CI: 0.62, 1.79); P=0.85* |
Letrozole tablets 2.5: aminoglutethimide=1.61 (95% CI: 0.90, 2.87); P=0.11* |
|
|
Relative Risk of Progression
|
Letrozole tablets 2.5: Letrozole tablets 0.5=0.86 (95% CI: 0.68, 1.11); P=0.25* |
Letrozole tablets 2.5: aminoglutethimide=0.74 (95% CI: 0.57, 0.94); P=0.02* |
|
*two-sided P-value
Figure 5 Kaplan-Meier Estimates of Time to Progression (Aminoglutethimide Study)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Information for Patients
Letrozole tablets 2.5 mg-Bottle of 30 tablets
Keep this and all the drugs out of reach of children.

Letrozole tablets 2.5 mg-Bottle of 1000 tablets
Keep this and all the drugs out of reach of children.

LETROZOLELETROZOLE TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||