LETROZOLE
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Letrozole Tablets, USP safely and effectively. See full prescribing information for Letrozole Tablets, USP Letrozole Tablets, USPInitial U.S. Approval: 1997 RECENT MAJOR CHANGES1.12.2INDICATIONS AND USAGE Adjuvant treatment of postmenopausal women with hormone receptor positive early breast cancer (1.1) Extended adjuvant treatment of postmenopausal women with early breast cancer who have received prior standard adjuvant tamoxifen therapy (1.2) First and second-line treatment of postmenopausal women with hormone receptor positive or unknown advanced breast cancer (1.3) DOSAGE AND ADMINISTRATION2 Recommended dose: 2.5 mg once daily (2.1) Patients with cirrhosis or severe hepatic impairment: 2.5 mg every other day (2.5, 5.3) DOSAGE FORMS AND STRENGTHS3CONTRAINDICATIONS4WARNINGS AND PRECAUTIONS Decreases in bone mineral density may occur. Consider bone mineral density monitoring (5.1) Increases in total cholesterol may occur. Consider cholesterol monitoring. (5.2) Fatigue, dizziness and somnolence may occur. Exercise caution when operating machinery (5.4) Side Effects6.16.26.36.4To report SUSPECTED ADVERSE REACTIONS, contact APP Pharmaceuticals, LLC, Medical Affairs at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 INDICATIONS & USAGE
- 2 DOSAGE & ADMINISTRATION
- 3 DOSAGE FORMS & STRENGTHS
- 4 LETROZOLE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 LETROZOLE ADVERSE REACTIONS
- 6.1 Adjuvant Treatment of Early Breast Cancer
- 6.2 Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 24 months
- 6.3 Updated Analysis, Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 60 Months
- 6.4 First-Line Treatment of Advanced Breast Cancer
- 6.5 Second- Line Treatment of Advanced Breast Cancer
- 6.6 First and Second-Line Treatment of Advanced Breast Cancer
- 6.7 Postmarketing Experience
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 LETROZOLE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 14.1 Updated Adjuvant Treatment of Early Breast Cancer
- 14.2 Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 24 Months
- 14.3 Updated Analyses of Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 60 Months
- 14.4 First-Line Treatment of Advanced Breast Cancer
- 14.5 Second-Line Treatment of Advanced Breast Cancer
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS & USAGE
1.1 Adjuvant Treatment of Early Breast Cancer
1.2 Extended Adjuvant Treatment of Early Breast Cancer
[see Clinical Studies (14.2, 14.3)]
1.3 First and Second-Line Treatment of Advanced Breast Cancer
[see Clinical Studies (14.4, 14.5)].
2 DOSAGE & ADMINISTRATION
2.1 Recommended Dose
2.2 Use in Adjuvant Treatment of Early Breast Cancer
In the adjuvant setting, the optimal duration of treatment with letrozole is unknown. The planned duration of treatment in the study was 5 years with 73% of the patients having completed adjuvant therapy. Treatment should be discontinued at relapse [see Clinical Studies (14.1) ].
2.3 Use in Extended Adjuvant Treatment of Early Breast Cancer
In the extended adjuvant setting, the optimal treatment duration with letrozole tablets, USP is not known. The planned duration of treatment in the study was 5 years. In the final updated analysis, conducted at a median follow-up of 62 months, the median treatment duration was 60 months. Seventy-one percent of patients were treated for at least 3 years and 58% of patients completed least 4.5 years of extended adjuvant treatment. The treatment should be discontinued at tumor relapse [see Clinical Studies (14.2)].
2.4 Use in First and Second-Line Treatment of Advanced Breast Cancer
[see Clinical Studies (14.4, 14.5)]
2.5 Use in Hepatic Impairment
[see Warnings and Precautions (5.3)].
2.6 Use in Renal Impairment
[see Clinical Pharmacology (12.3)]
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
[see Use in Specific Populations (8.1)]
5 WARNINGS AND PRECAUTIONS
5.1 Bone Effects
Use of letrozole tablets, USP may cause decreases in bone mineral density (BMD). Consideration should be given to monitoring BMD. Results of a substudy to evaluate safety in the adjuvant setting comparing the effect on lumbar spine (L2-L4) bone mineral density (BMD) of adjuvant treatment with letrozole to that with tamoxifen showed at 24 months a median decrease in lumbar spine BMD of 4.1% in the letrozole arm compared to a median increase of 0.3% in the tamoxifen arm (difference = 4.4%) (P<0.0001) [See Adverse reactions (6.1)]. Updated results from the BMD sub-study in the extended adjuvant setting demonstrated that at 2 years patients receiving letrozole had a median decrease from baseline of 3.8% in hip BMD compared to a median decrease of 2.0% in the placebo group. The changes from baseline in lumbar spine BMD in letrozole and placebo treated groups were not significantly different [see Adverse Reactions (6.2)].
In the adjuvant trial the incidence of bone fractures at any time after randomization was 13.8% for letrozole and 10.5% for tamoxifen. The incidence of osteoporosis was 5.1% for letrozole and 2.7% for tamoxifen [See Adverse Reactions (6.1)]. In the extended adjuvant trial the incidence of bone fractures at any time after randomization was 13.3% for letrozole and 7.8% for placebo. The incidence of new osteoporosis was 14.5% for letrozole and 7.8% for placebo [see Adverse Reactions (6.3)].
5.2 Cholesterol
Consideration should be given to monitoring serum cholesterol. In the adjuvant trial hypercholesterolemia was reported in 52.3% of letrozole patients and 28.6% of tamoxifen patients. CTC grade 3-4 hypercholesterolemia was reported in 0.4% of letrozole patients and 0.1% of tamoxifen patients. Also in the adjuvant setting, an increase of ≥1.5 X ULN in total cholesterol (generally non-fasting) was observed in patients on monotherapy who had baseline total serum cholesterol within the normal range (i.e., <=1.5 X ULN) in 151/1843 (8.2%) on letrozole vs 57/1840 (3.2%). Lipid lowering medications were required for 25% of patients on letrozole and 16% on tamoxifen [see Adverse Reactions (6.1)].
5.3 Hepatic Impairment
[see Dosage and Administration (2.5)].
5.4 Fatigue and Dizziness
5.5 Laboratory Test Abnormalities
6 ADVERSE REACTIONS
The most serious adverse reactions from the use of letrozole tablets, USP are:
- Bone effects [see Warnings and Precautions ( 5.1 )]
- Increases in cholesterol [see Warnings and Precautions ( 5.2 )]
6.1 Adjuvant Treatment of Early Breast Cancer
Table 1: Patients with Adverse Reactions (CTC Grades 1-4, Irrespective of Relationship to Study Drug) in the Adjuvant Study – Monotherapy Arms Analysis (Median Follow-up 73 Months; Median Treatment 60 Months)
|
|
Grades 1-4 |
Grades 3-4 |
||||||
|
Adverse Reaction |
Letrozole tablets, USP N=2448 n (%) |
tamoxifen N=2447 n (%) |
Letrozole tablets, USP N=2448 n (%) |
tamoxifen N=2447 n (%) |
||||
|
Pts with any adverse event |
2310 |
(94.4) |
2214 |
(90.5) |
635 |
(25.9) |
604 |
(24.7) |
|
Hypercholesterolemia |
1280 |
(52.3) |
700 |
(28.6) |
11 |
(0.4) |
6 |
(0.2) |
|
Hot Flashes/Flushes |
821 |
(33.5) |
929 |
(38) |
0 |
- |
0 |
- |
|
Arthralgia/Arthritis |
618 |
(25.2) |
501 |
(20.4) |
85 |
(3.5) |
50 |
(2) |
|
Night Sweats |
357 |
(14.6) |
426 |
(17.4) |
0 |
- |
0 |
- |
|
Bone Fractures  |
338 |
(13.8) |
257 |
(10.5) |
- |
- |
- |
- |
|
Weight Increase |
317 |
(12.9) |
378 |
(15.4) |
27 |
(1.1) |
39 |
(1.6) |
|
Nausea |
283 |
(11.6) |
277 |
(11.3) |
6 |
(0.2) |
9 |
(0.4) |
|
Bone Fractures  |
247 |
(10.1) |
174 |
(7.1) |
- |
- |
- |
- |
|
Fatigue (Lethargy, Malaise, Asthenia) |
235 |
(9.6) |
250 |
(10.2) |
6 |
(0.2) |
7 |
(0.3) |
|
Myalgia |
217 |
(8.9) |
212 |
(8.7) |
18 |
(0.7) |
14 |
(0.6) |
|
Edema |
164 |
(6.7) |
160 |
(6.5) |
3 |
(0.1) |
1 |
(<0.1) |
|
Weight Decrease |
140 |
(5.7) |
129 |
(5.3) |
8 |
(0.3) |
5 |
(0.2) |
|
Vaginal Bleeding |
128 |
(5.2) |
320 |
(13.1) |
1 |
(<0.1) |
8 |
(0.3) |
|
Back Pain |
125 |
(5.1) |
136 |
(5.6) |
7 |
(0.3) |
11 |
(0.4) |
|
Osteoporosis NOS |
124 |
(5.1) |
66 |
(2.7) |
10 |
(0.4) |
5 |
(0.2) |
|
Bone pain |
123 |
(5) |
109 |
(4.5) |
6 |
(0.2) |
4 |
(0.2) |
|
Depression |
119 |
(4.9) |
114 |
(4.7) |
16 |
(0.7) |
14 |
(0.6) |
|
Vaginal Irritation |
111 |
(4.5) |
77 |
(3.1) |
2 |
(<0.1) |
2 |
(<0.1) |
|
Headache |
105 |
(4.3) |
94 |
(3.8) |
9 |
(0.4) |
5 |
(0.2) |
|
Pain in extremity |
103 |
(4.2) |
79 |
(3.2) |
6 |
(0.2) |
4 |
(0.2) |
|
Osteopenia |
87 |
(3.6) |
74 |
(3) |
0 |
- |
2 |
(<0.1) |
|
Dizziness/Light-Headedness |
84 |
(3.4) |
84 |
(3.4) |
1 |
(<0.1) |
6 |
(0.2) |
|
Alopecia |
83 |
(3.4) |
84 |
(3.4) |
0 |
- |
0 |
- |
|
Vomiting |
80 |
(3.3) |
80 |
(3.3) |
3 |
(0.1) |
5 |
(0.2) |
|
Cataract |
49 |
(2) |
54 |
(2.2) |
16 |
(0.7) |
17 |
(0.7) |
|
Constipation |
49 |
(2) |
71 |
(2.9) |
3 |
(0.1) |
1 |
(<0.1) |
|
Breast pain |
37 |
(1.5) |
43 |
(1.8) |
1 |
(<0.1) |
0 |
- |
|
Anorexia |
20 |
(0.8) |
20 |
(0.8) |
1 |
(<0.1) |
1 |
(<0.1) |
|
Endometrial Hyperplasia/Cancer   |
11/1909 |
(0.6) |
70/1943 |
(3.6) |
- |
- |
- |
- |
|
Endometrial Proliferation Disorders |
10 |
(0.3) |
71 |
(1.8) |
0 |
- |
14 |
(0.6) |
|
Endometrial Hyperplasia/Cancer   |
6/1909 |
(0.3) |
57/1943 |
(2.9) |
- |
- |
- |
- |
|
Other Endometrial Disorders |
2 |
(<0.1) |
3 |
(0.1) |
0 |
- |
0 |
- |
|
Myocardial Infarction  |
24 |
(1) |
12 |
(0.5) |
- |
- |
- |
- |
|
Myocardial Infarction  |
37 |
(1.5) |
25 |
(1) |
- |
- |
- |
- |
|
Myocardial Ischemia |
6 |
(0.2) |
9 |
(0.4) |
- |
- |
- |
- |
|
Cerebrovascular Accident  |
52 |
(2.1) |
46 |
(1.9) |
- |
- |
- |
- |
|
Cerebrovascular Accident  |
70 |
(2.9) |
63 |
(2.6) |
- |
- |
- |
- |
|
Angina  |
26 |
(1.1) |
24 |
(1) |
- |
- |
- |
- |
|
Angina  |
32 |
(1.3) |
31 |
(1.3) |
- |
- |
- |
- |
|
Thromboembolic Event  |
51 |
(2.1) |
89 |
(3.6) |
- |
- |
- |
- |
|
Thromboembolic Event  |
71 |
(2.9) |
111 |
(4.5) |
- |
- |
- |
- |
|
Other Cardiovascular  |
260 |
(10.6) |
256 |
(10.5) |
- |
- |
- |
- |
|
Other Cardiovascular  |
312 |
(12.7) |
337 |
(13.8) |
- |
- |
- |
- |
|
Second Malignancies  |
53 |
(2.2) |
78 |
(3.2) |
- |
- |
- |
- |
|
Second Malignancies  |
102 |
(4.2) |
119 |
(4.9) |
- |
- |
- |
- |
P
6.2 Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 24 months
The median duration of extended adjuvant treatment was 24 months and the median duration of follow-up for safety was 28 months for patients receiving letrozole tablets, USP and placebo.
Table 2 describes the adverse reactions occurring at a frequency of at least 5% in any treatment group during treatment. Most adverse reactions reported were Grade 1 and Grade 2 based on the Common Toxicity Criteria Version 2.0. In the extended adjuvant setting, the reported drug-related adverse reactions that were significantly different from placebo were hot flashes, arthralgia/arthritis, and myalgia.
Table 2: Percentage of Patients with Adverse Reactions
| Number (%) of Patients with Grade 1-4 Adverse Reaction | Number (%) of Patients with Grade 3-4 Adverse Reaction | |||
|---|---|---|---|---|
| Letrozole Tablets, USP N=2563 |
Placebo N=2573 |
Letrozole Tablets, USP N=2563 |
Placebo N=2573 |
|
|
Any Adverse Reaction
|
2232 (87.1) |
2174 (84.5) |
419 (16.3) |
389 (15.1) |
|
Vascular Disorders
|
1375 (53.6) |
1230 (47.8) |
59 (2.3) |
74 (2.9) |
| Flushing |
1273 (49.7) |
1114 (43.3) |
3 (0.1) |
0 - |
|
General Disorders
|
1154 (45) |
1090 (42.4) |
30 (1.2) |
28 (1.1) |
| Asthenia |
862 (33.6) |
826 (32.1) |
16 (0.6) |
7 (0.3) |
| Edema NOS |
471 (18.4) |
416 (16.2) |
4 (0.2) |
3 (0.1) |
|
Musculoskeletal Disorders
|
978 (38.2) |
836 (32.5) |
71 (2.8) |
50 (1.9) |
| Arthralgia |
565 (22) |
465 (18.1) |
25 (1) |
20 (0.8) |
| Arthritis NOS |
173 (6.7) |
124 (4.8) |
10 (0.4) |
5 (0.2) |
| Myalgia |
171 (6.7) |
122 (4.7) |
8 (0.3) |
6 (0.2) |
| Back Pain |
129 (5) |
112 (4.4) |
8 (0.3) |
7 (0.3) |
|
Nervous System Disorders
|
863 (33.7) |
819 (31.8) |
65 (2.5) |
58 (2.3) |
| Headache |
516 (20.1) |
508 (19.7) |
18 (0.7) |
17 (0.7) |
| Dizziness |
363 (14.2) |
342 (13.3) |
9 (0.4) |
6 (0.2) |
|
Skin Disorders
|
830 (32.4) |
787 (30.6) |
17 (0.7) |
16 (0.6) |
| Sweating Increased |
619 (24.2) |
577 (22.4) |
1 (<0.1) |
0 - |
|
Gastrointestinal Disorders
|
725 (28.3) |
731 (28.4) |
43 (1.7) |
42 (1.6) |
| Constipation |
290 (11.3) |
304 (11.8) |
6 (0.2) |
2 (<0.1) |
| Nausea |
221 (8.6) |
212 (8.2) |
3 (0.1) |
10 (0.4) |
| Diarrhea NOS |
128 (5) |
143 (5.6) |
12 (0.5) |
8 (0.3) |
|
Metabolic Disorders
|
551 (21.5) |
537 (20.9) |
24 (0.9) |
32 (1.2) |
| Hypercholesterolemia |
401 (15.6) |
398 (15.5) |
2 (<0.1) |
5 (0.2) |
|
Reproductive Disorders
|
303 (11.8) |
357 (13.9) |
9 (0.4) |
8 (0.3) |
| Vaginal Hemorrhage |
123 (4.8) |
171 (6.6) |
2 (<0.1) |
5 (0.2) |
| Vulvovaginal Dryness |
137 (5.3) |
127 (4.9) |
0 - |
0 - |
|
Psychiatric Disorders
|
320 (12.5) |
276 (10.7) |
21 (0.8) |
16 (0.6) |
| Insomnia |
149 (5.8) |
120 (4.7) |
2 (<0.1) |
2 (<0.1) |
|
Respiratory Disorders
|
279 (10.9) |
260 (10.1) |
30 (1.2) |
28 (1.1) |
| Dyspnea |
140 (5.5) |
137 (5.3) |
21 (0.8) |
18 (0.7) |
|
Investigations
|
184 (7.2) |
147 (5.7) |
13 (0.5) |
13 (0.5) |
|
Infections and Infestations
|
166 (6.5) |
163 (6.3) |
40 (1.6) |
33 (1.3) |
|
Renal Disorders
|
130 (5.1) |
100 (3.9) |
12 (0.5) |
6 (0.2) |
Based on a median follow-up of patients for 28 months, the incidence of clinical fractures from the core randomized study in patients who received letrozole tablets, USP was 5.9% (152) and placebo was 5.5% (142). The incidence of self-reported osteoporosis was higher in patients who received letrozole tablets, USP 6.9% (176) than in patients who received placebo 5.5% (141). Bisphosphonates were administered to 21.1% of the patients who received letrozole tablets, USP and 18.7% of the patients who received placebo.
The incidence of cardiovascular ischemic events from the core randomized study was comparable between patients who received letrozole tablets, USP 6.8% (175) and placebo 6.5% (167).
A patient-reported measure that captures treatment impact on important symptoms associated with estrogen deficiency demonstrated a difference in favor of placebo for vasomotor and sexual symptom domains.
Bone Sub-study: [see Warnings and Precautions ( 5.1 )].
Lipid Sub-study: In the extended adjuvant setting, based on a median duration of follow-up of 62 months, there was no significant difference between letrozole tablets, USP and placebo in total cholesterol or in any lipid fraction at any time over 5 years. Use of lipid lowering drugs or dietary management of elevated lipids was allowed. [see Warnings and Precautions ( 5.2 )]
6.3 Updated Analysis, Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 60 Months
[see Adverse Reactions (6.2)]
[see Warnings and Precautions(5.2)]
6.4 First-Line Treatment of Advanced Breast Cancer
A total of 455 patients were treated for a median time of exposure of 11 months. The incidence of adverse reactions was similar for letrozole tablets, USP and tamoxifen. The most frequently reported adverse reactions were bone pain, hot flushes, back pain, nausea, arthralgia and dyspnea. Discontinuations for adverse reactions other than progression of tumor occurred in 10/455 (2%) of patients on letrozole tablets, USP and in 15/455 (3%) of patients on tamoxifen.
Adverse reactions, regardless of relationship to study drug, that were reported in at least 5% of the patients treated with letrozole tablets, USP 2.5 mg or tamoxifen 20 mg in the first-line treatment study are shown in Table 3.
Table 3: Percentage (%) of Patients with Adverse Reactions
| Adverse Reaction |
Letrozole Tablets, USP 2.5 mg (N=455) % |
tamoxifen 20 mg (N=455)% |
|---|---|---|
|
General Disorders
|
||
| Fatigue |
13 |
13 |
| Chest Pain |
8 |
9 |
| Edema Peripheral |
5 |
6 |
| Pain NOS |
5 |
7 |
| Weakness |
6 |
4 |
|
Investigations
|
||
| Weight Decreased |
7 |
5 |
|
Vascular Disorders
|
||
| Hot Flushes |
19 |
16 |
| Hypertension |
8 |
4 |
|
Gastrointestinal Disorders
|
||
| Nausea |
17 |
17 |
| Constipation |
10 |
11 |
| Diarrhea |
8 |
4 |
| Vomiting |
7 |
8 |
|
Infections/Infestations
|
||
| Influenza |
6 |
4 |
| Urinary Tract Infection NOS |
6 |
3 |
|
Injury, Poisoning and Procedural Complications
|
||
| Post-Mastectomy Lymphedema |
7 |
7 |
|
Metabolism and Nutrition Disorders
|
||
| Anorexia |
4 |
6 |
|
Musculoskeletal and Connective Tissue Disorders
|
||
| Bone Pain |
22 |
21 |
| Back Pain |
18 |
19 |
| Arthralgia |
16 |
15 |
| Pain in Limb |
10 |
8 |
|
Nervous System Disorders
|
||
| Headache NOS |
8 |
7 |
|
Psychiatric Disorders
|
||
| Insomnia |
7 |
4 |
|
Reproductive System and Breast Disorders
|
||
| Breast Pain |
7 |
7 |
|
Respiratory, Thoracic and Mediastinal Disorders
|
||
| Dyspnea |
18 |
17 |
| Cough |
13 |
13 |
| Chest Wall Pain |
6 |
6 |
6.5 Second- Line Treatment of Advanced Breast Cancer
Study discontinuations in the megestrol acetate comparison study for adverse reactions other than progression of tumor were 5/188 (2.7%) on letrozole tablets, USP 0.5 mg, in 4/174 (2.3%) on letrozole tablets, USP 2.5 mg, and in 15/190 (7.9%) on megestrol acetate. There were fewer thromboembolic events at both letrozole tablets, USP doses than on the megestrol acetate arm (0.6% vs 4.7%). There was also less vaginal bleeding (0.3% vs 3.2%) on letrozole tablets, USP than on megestrol acetate. In the aminoglutethimide comparison study, discontinuations for reasons other than progression occurred in 6/193 (3.1%) on 0.5 mg letrozole tablets, USP 7/185 (3.8%) on 2.5 mg letrozole tablets, USP and 7/178 (3.9%) of patients on aminoglutethimide.
Comparisons of the incidence of adverse reactions revealed no significant differences between the high and low dose letrozole tablets, USP groups in either study. Most of the adverse reactions observed in all treatment groups were mild to moderate in severity and it was generally not possible to distinguish adverse reactions due to treatment from the consequences of the patient’s metastatic breast cancer, the effects of estrogen deprivation, or intercurrent illness.
Adverse reactions, regardless of relationship to study drug, that were reported in at least 5% of the patients treated with letrozole tablets, USP 0.5 mg, letrozole tablets, USP 2.5 mg, megestrol acetate, or aminoglutethimide in the two controlled trials are shown in Table 4.
Table 4: Percentage (%) of Patients with Adverse Reactions
| Adverse Reaction | Pooled Letrozole Tablets, USP 2.5 mg (N=359) % |
Pooled Letrozole Tablets, USP 0.5 mg (N=380) % |
megestrol acetate 160 mg (N=189) % |
aminoglutethimide 500 mg (N=178) % |
|---|---|---|---|---|
|
Body as a Whole
|
||||
| Fatigue |
8 |
6 |
11 |
3 |
| Chest Pain |
6 |
3 |
7 |
3 |
Peripheral Edema |
5 |
5 |
8 |
3 |
| Asthenia |
4 |
5 |
4 |
5 |
| Weight Increase |
2 |
2 |
9 |
3 |
|
Cardiovascular
|
||||
| Hypertension |
5 |
7 |
5 |
6 |
|
Digestive System
|
||||
| Nausea |
13 |
15 |
9 |
14 |
| Vomiting |
7 |
7 |
5 |
9 |
| Constipation |
6 |
7 |
9 |
7 |
| Diarrhea |
6 |
5 |
3 |
4 |
| Pain-Abdominal |
6 |
5 |
9 |
8 |
| Anorexia |
5 |
3 |
5 |
5 |
| Dyspepsia |
3 |
4 |
6 |
5 |
|
Infections/Infestations
|
||||
| Viral Infection |
6 |
5 |
6 |
3 |
|
Lab Abnormality
|
||||
| Hypercholesterolemia |
3 |
3 |
0 |
6 |
|
Musculoskeletal System
|
||||
Musculoskeletal |
21 |
22 |
30 |
14 |
| Arthralgia |
8 |
8 |
8 |
3 |
|
Nervous System
|
||||
| Headache |
9 |
12 |
9 |
7 |
| Somnolence |
3 |
2 |
2 |
9 |
| Dizziness |
3 |
5 |
7 |
3 |
|
Respiratory System
|
||||
| Dyspnea |
7 |
9 |
16 |
5 |
| Coughing |
6 |
5 |
7 |
5 |
|
Skin and Appendages
|
||||
| Hot Flushes |
6 |
5 |
4 |
3 |
Rash |
5 |
4 |
3 |
12 |
| Pruritus |
1 |
2 |
5 |
3 |
Other less frequent (<5%) adverse reactions considered consequential and reported in at least 3 patients treated with letrozole tablets, USP, included hypercalcemia, fracture, depression, anxiety, pleural effusion, alopecia, increased sweating and vertigo.
6.6 First and Second-Line Treatment of Advanced Breast Cancer
6.7 Postmarketing Experience
7 DRUG INTERACTIONS
Tamoxifen
Coadministration of letrozole tablets, USP and tamoxifen 20 mg daily resulted in a reduction of letrozole plasma levels of 38% on average. Clinical experience in the second-line breast cancer trials indicates that the therapeutic effect of letrozole tablets, USP therapy is not impaired if letrozole tablets, USP is administered immediately after tamoxifen.
Cimetidin e
A pharmacokinetic interaction study with cimetidine showed no clinically significant effect on letrozole pharmacokinetics.
Warfarin
An interaction study with warfarin showed no clinically significant effect of letrozole on warfarin pharmacokinetics.
Other anticancer agents
There is no clinical experience to date on the use of letrozole tablets, USP in combination with other anticancer agents.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category X [see Contraindications (4)]
2
222see Nonclinical Toxicology (13.2
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
The median age of patients in all studies of first-line and second-line treatment of metastatic breast cancer was 64-65 years. About 1/3 of the patients were ≥ 70 years old. In the first-line study, patients ≥ 70 years of age experienced longer time to tumor progression and higher response rates than patients <70.
For the extended adjuvant setting, more than 5,100 postmenopausal women were enrolled in the clinical study. In total, 41% of patients were aged 65 years or older at enrollment, while 12% were 75 or older. In the extended adjuvant setting, no overall differences in safety or efficacy were observed between these older patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
In the adjuvant setting, more than 8,000 postmenopausal women were enrolled in the clinical study. In total, 36 % of patients were aged 65 years or older at enrollment, while 12% were 75 or older. More adverse reactions were generally reported in elderly patients irrespective of study treatment allocation. However, in comparison to tamoxifen, no overall differences with regards to the safety and efficacy profiles were observed between elderly patients and younger patients.
10 OVERDOSAGE
Isolated cases of letrozole tablets, USP overdose have been reported. In these instances, the highest single dose ingested was 62.5 mg or 25 tablets. While no serious adverse reactions were reported in these cases, because of the limited data available, no firm recommendations for treatment can be made. However, emesis could be induced if the patient is alert. In general, supportive care and frequent monitoring of vital signs are also appropriate. In single-dose studies, the highest dose used was 30 mg, which was well tolerated; in multiple-dose trials, the largest dose of 10 mg was well tolerated.
2211 DESCRIPTION

17115
Inactive Ingredients:
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The growth of some cancers of the breast is stimulated or maintained by estrogens. Treatment of breast cancer thought to be hormonally responsive (i.e., estrogen and/or progesterone receptor positive or receptor unknown) has included a variety of efforts to decrease estrogen levels (ovariectomy, adrenalectomy, hypophysectomy) or inhibit estrogen effects (antiestrogens and progestational agents). These interventions lead to decreased tumor mass or delayed progression of tumor growth in some women.
In postmenopausal women, estrogens are mainly derived from the action of the aromatase enzyme, which converts adrenal androgens (primarily androstenedione and testosterone) to estrone and estradiol. The suppression of estrogen biosynthesis in peripheral tissues and in the cancer tissue itself can therefore be achieved by specifically inhibiting the aromatase enzyme.
Letrozole is a nonsteroidal competitive inhibitor of the aromatase enzyme system; it inhibits the conversion of androgens to estrogens. In adult nontumor- and tumor-bearing female animals, letrozole is as effective as ovariectomy in reducing uterine weight, elevating serum LH, and causing the regression of estrogen-dependent tumors. In contrast to ovariectomy, treatment with letrozole does not lead to an increase in serum FSH. Letrozole selectively inhibits gonadal steroidogenesis but has no significant effect on adrenal mineralocorticoid or glucocorticoid synthesis.
12.2 Pharmacodynamics
In postmenopausal patients with advanced breast cancer, daily doses of 0.1 mg to 5 mg letrozole tablet, USP suppress plasma concentrations of estradiol, estrone, and estrone sulfate by 75%-95% from baseline with maximal suppression achieved within two-three days. Suppression is dose-related, with doses of 0.5 mg and higher giving many values of estrone and estrone sulfate that were below the limit of detection in the assays. Estrogen suppression was maintained throughout treatment in all patients treated at 0.5 mg or higher.
Letrozole is highly specific in inhibiting aromatase activity. There is no impairment of adrenal steroidogenesis. No clinically-relevant changes were found in the plasma concentrations of cortisol, aldosterone, 11-deoxycortisol, 17-hydroxy-progesterone, ACTH or in plasma renin activity among postmenopausal patients treated with a daily dose of letrozole tablets, USP 0.1 mg to 5 mg. The ACTH stimulation test performed after 6 and 12 weeks of treatment with daily doses of 0.1, 0.25, 0.5, 1, 2.5, and 5 mg did not indicate any attenuation of aldosterone or cortisol production. Glucocorticoid or mineralocorticoid supplementation is, therefore, not necessary.
12.3 Pharmacokinetics
Absorption and Distribution:Letrozole is rapidly and completely absorbed from the gastrointestinal tract and absorption is not affected by food. It is metabolized slowly to an inactive metabolite whose glucuronide conjugate is excreted renally, representing the major clearance pathway. About 90% of radiolabeled letrozole is recovered in urine. Letrozole’s terminal elimination half-life is about 2 days and steady-state plasma concentration after daily 2.5 mg dosing is reached in 2-6 weeks. Plasma concentrations at steady state are 1.5 to 2 times higher than predicted from the concentrations measured after a single dose, indicating a slight non-linearity in the pharmacokinetics of letrozole upon daily administration of 2.5 mg. These steady-state levels are maintained over extended periods, however, and continuous accumulation of letrozole does not occur. Letrozole is weakly protein bound and has a large volume of distribution (approximately 1.9 L/kg).
Metabolism and Excretion:Metabolism to a pharmacologically-inactive carbinol metabolite (4, 4'-methanol-bisbenzonitrile) and renal excretion of the glucuronide conjugate of this metabolite is the major pathway of letrozole clearance. Of the radiolabel recovered in urine, at least 75% was the glucuronide of the carbinol metabolite, about 9% was two unidentified metabolites, and 6% was unchanged letrozole.
In human microsomes with specific CYP isozyme activity, CYP3A4 metabolized letrozole to the carbinol metabolite while CYP2A6 formed both this metabolite and its ketone analog. In human liver microsomes, letrozole strongly inhibited CYP2A6 and moderately inhibited CYP2C19.
Pediatric, Geriatric and Race: In the study populations (adults ranging in age from 35 to >80 years), no change in pharmacokinetic parameters was observed with increasing age. Differences in letrozole pharmacokinetics between adult and pediatric populations have not been studied. Differences in letrozole pharmacokinetics due to race have not been studied.
Renal Impairment: In a study of volunteers with varying renal function (24-hour creatinine clearance: 9-116 mL/min), no effect of renal function on the pharmacokinetics of single doses of 2.5 mg of letrozole tablets, USP was found. In addition, in a study of 347 patients with advanced breast cancer, about half of whom received 2.5 mg letrozole tablets, USP and half 0.5 mg letrozole tablets, USP, renal impairment (calculated creatinine clearance: 20-50 mL/min) did not affect steady-state plasma letrozole concentrations.
Hepatic Impairment: In a study of subjects with mild to moderate non-metastatic hepatic dysfunction (e.g., cirrhosis, Child-Pugh classification A and B), the mean AUC values of the volunteers with moderate hepatic impairment were 37% higher than in normal subjects, but still within the range seen in subjects without impaired function.
In a pharmacokinetic study, subjects with liver cirrhosis and severe hepatic impairment (Child-Pugh classification C, which included bilirubins about 2-11 times ULN with minimal to severe ascites) had two-fold increase in exposure (AUC) and 47% reduction in systemic clearance. Breast cancer patients with severe hepatic impairment are thus expected to be exposed to higher levels of letrozole than patients with normal liver function receiving similar doses of this drug. [see Dosage and Administration ( 2.5 )]
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
20-12hr0-24hr20-24hr
in vitroin vitroin vivo
2
13.2 Animal Pharmacology & Or Toxicology
Reproductive Toxicology: 22
2
14 CLINICAL STUDIES
14.1 Updated Adjuvant Treatment of Early Breast Cancer
In a multicenter study enrolling over 8,000 postmenopausal women with resected, receptor-positive early breast cancer, one of the following treatments was randomized in a double-blind manner:
Option 1:
A. tamoxifen for 5 years
B. letrozole tablets, USP for 5 years
C. tamoxifen for 2 years followed by letrozole tablets, USP for 3 years
D. letrozole tablets, USP for 2 years followed by tamoxifen for 3 years
Option 2:
A. tamoxifen for 5 years
B. letrozole tablets, USP for 5 years
The study in the adjuvant setting, BIG 1-98 was designed to answer two primary questions: whether letrozole tablets, USP for 5 years was superior to tamoxifen for 5 years (Primary Core Analysis) and whether switching endocrine treatments at 2 years was superior to continuing the same agent for a total of 5 years (Sequential Treatments Analysis). Selected baseline characteristics for the study population are shown in Table 5. The primary endpoint of this trial was disease-free survival (DFS) (i.e., interval between randomization and earliest occurrence of a local, regional, or distant recurrence, or invasive contralateral breast cancer, or death from any cause). The secondary endpoints were overall survival (OS), systemic disease-free survival (SDFS), invasive contralateral breast cancer, time to breast cancer recurrence (TBR) and time to distant metastasis (TDM).
The Primary Core Analysis (PCA) included all patients and all follow-up in the monotherapy arms in both randomization options, but follow-up in the two sequential treatments arms was truncated 30 days after switching treatments. The PCA was conducted at a median treatment duration of 24 months and a median follow-up of 26 months. Letrozole tablets, USP was superior to tamoxifen in all endpoints except overall survival and contralateral breast cancer [e.g., DFS: hazard ratio, HR 0.79; 95% CI (0.68, 0.92); P=0.002; SDFS: HR 0.83; 95% CI (0.70, 0.97); TDM: HR 0.73; 95% CI (0.60, 0.88); OS: HR 0.86; 95% CI (0.70, 1.06).
In 2005, based on recommendations by the independent Data Monitoring Committee, the tamoxifen arms were unblinded and patients were allowed to complete initial adjuvant therapy with letrozole tablets, USP (if they had received tamoxifen for at least 2 years) or to start extended adjuvant treatment with letrozole tablets, USP (if they had received tamoxifen for at least 4.5 years) if they remained alive and disease-free. In total, 632 patients crossed to letrozole tablets, USP or another aromatase inhibitor. Approximately 70% (448) of these 632 patients crossed to letrozole tablets, USP to complete initial adjuvant therapy and most of these crossed in years 3 to 4. All of these patients were in Option 1. A total of 184 patients started extended adjuvant therapy with letrozole tablets, USP (172 patients) or with another aromatase inhibitor (12 patients). To explore the impact of this selective crossover, results from analyses censoring follow-up at the date of the selective crossover (in the tamoxifen arm) are presented for the Monotherapy Arms Analysis (MAA).
The PCA allowed the results of letrozole tablets, USP for 5 years compared with tamoxifen for 5 years to be reported in 2005 after a median follow-up of only 26 months. The design of the PCA is not optimal to evaluate the effect of letrozole tablets, USP after a longer time (because follow-up was truncated in two arms at around 25 months). The Monotherapy Arms Analysis (ignoring the two sequential treatment arms) provided follow-up equally as long in each treatment and did not over-emphasize early recurrences as the PCA did. The MAA thus provides the clinically appropriate updated efficacy results in answer to the first primary question, despite the confounding of the tamoxifen reference arm by the selective crossover to letrozole tablets, USP. The updated results for the MAA are summarized in Table 6. Median follow-up for this analysis is 73 months.
The Sequential Treatments Analysis (STA) addresses the second primary question of the study. The primary analysis for the Sequential Treatments Analysis (STA) was from switch (or equivalent time-point in monotherapy arms) + 30 days (STA-S) with a two-sided test applied to each pair-wise comparison at the 2.5% level. Additional analyses were conducted from randomization (STA-R) but these comparisons (added in light of changing medical practice) were under-powered for efficacy.
Table 5: Adjuvant Study - Patient and Disease Characteristics (ITT Population)
|
Characteristic |
Primary Core Analysis (PCA)
|
Monotherapy Arms Analysis (MAA)
|
||
|
Letrozole tablets, USP
N=4003 n (%) |
tamoxifen
N=4007 n (%) |
Letrozole tablets, USP
N=2463 n (%) |
tamoxifen
N=2459 n (%) |
|
| Age (median, years) |
61 |
61 |
61 |
61 |
| Age range (years) |
38-89 |
39-90 |
38-88 |
39-90 |
| Hormone receptor status (%) |
|
|
|
|
| ER+ and/or PgR+ |
99.7 |
99.7 |
99.7 |
99.7 |
| Both unknown |
0.3 |
0.3 |
0.3 |
0.3 |
| Nodal status (%) |
|
|
|
|
| Node negative |
52 |
52 |
50 |
52 |
| Node positive |
41 |
41 |
43 |
41 |
| Nodal status unknown |
7 |
7 |
7 |
7 |
| Prior adjuvant chemotherapy (%) |
24 |
24 |
24 |
24 |
Table 6: Updated Adjuvant Study Results - Monotherapy Arms Analysis (Median Follow-up 73 Months)
| |
Letrozole tablets, USP
N=2463 |
Tamoxifen
N=2459 |
Hazard ratio
|
|
|||
| |
Events (%)
|
5-year rate
|
Events (%)
|
5-year rate
|
(95% CI)
|
P
|
|
| Disease-free survival1
|
ITT |
445 (18.1) |
87.4 |
500 (20.3) |
84.7 |
0.87 (0.76, 0.99) |
0.03 |
|
|
Censor |
445 |
87.4 |
483 |
84.2 |
0.84 (0.73, 0.95) |
|
| 0 positive nodes |
ITT |
165 |
92.2 |
189 |
90.3 |
0.88 (0.72, 1.09) |
|
| 1-3 positive nodes |
ITT |
151 |
85.6 |
163 |
83 |
0.85 (0.68, 1.06) |
|
| >=4 positive nodes |
ITT |
123 |
71.2 |
142 |
62.6 |
0.81 (0.64, 1.03) |
|
| Adjuvant chemotherapy |
ITT |
119 |
86.4 |
150 |
80.6 |
0.77 (0.60, 0.98) |
|
| No chemotherapy |
ITT |
326 |
87.8 |
350 |
86.1 |
0.91 (0.78, 1.06) |
|
| Systemic DFS2
|
ITT |
401 |
88.5 |
446 |
86.6 |
0.88 (0.77,1.01) |
|
| Time to distant metastasis3 |
ITT |
257 |
92.4 |
298 |
90.1 |
0.85 (0.72, 1) |
|
| Adjuvant chemotherapy |
ITT |
84 |
- |
109 |
- |
0.75 (0.56-1) |
|
| No chemotherapy |
ITT |
173 |
- |
189 |
- |
0.90 (0.73,1.11) |
|
| Distant DFS4
|
ITT |
385 |
89 |
432 |
87.1 |
0.87 (0.76,1) |
|
| Contralateral breast cancer |
ITT |
34 |
99.2 |
44 |
98.6 |
0.76 (0.49, 1.19) |
|
| Overall survival |
ITT |
303 |
91.8 |
343 |
90.9 |
0.87 (0.75, 1.02) |
|
| |
Censor |
303 |
91.8 |
338 |
90.1 |
0.82 (0.70, 0.96) |
|
| 0 positive nodes |
ITT |
107 |
95.2 |
121 |
94.8 |
0.90 (0.69.1.16) |
|
| 1-3 positive nodes |
ITT |
99 |
90.8 |
114 |
90.6 |
0.81(0.62,1.06) |
|
| >=4 positive nodes |
ITT |
92 |
80.2 |
104 |
73.6 |
0.86 (0.65, 1.14) |
|
| Adjuvant chemotherapy |
ITT |
76 |
91.5 |
96 |
88.4 |
0.79 (0.58, 1.06) |
|
| No chemotherapy |
ITT |
227 |
91.9 |
247 |
91.8 |
0.91 (0.76, 1.08) |
|
1
2
3
4
Figure 1 Disease-Free Survival (Median follow-up 73 months, ITT Approach)

14.2 Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 24 Months
A double-blind, randomized, placebo-controlled trial of letrozole tablets, USP was performed in over 5,100 postmenopausal women with receptor-positive or unknown primary breast cancer who were disease free after 5 years of adjuvant treatment with tamoxifen.
The planned duration of treatment for patients in the study was 5 years, but the trial was terminated early because of an interim analysis showing a favorable letrozole tablets, USP effect on time without recurrence or contralateral breast cancer. At the time of unblinding, women had been followed for a median of 28 months, 30% of patients had completed 3 or more years of follow-up and less than 1% of patients had completed 5 years of follow-up.
Selected baseline characteristics for the study population are shown in Table 7.
Table 7: Selected Study Population Demographics (Modified ITT Population)
| Baseline Status | Letrozole Tablets, USP N=2582 |
Placebo N=2586 |
|---|---|---|
|
Hormone Receptor Status (%)
|
||
| ER+ and/or PgR+ |
98 |
98 |
| Both Unknown |
2 |
2 |
|
Nodal Status (%)
|
||
| Node Negative |
50 |
50 |
| Node Positive |
46 |
46 |
| Nodal Status Unknown |
4 |
4 |
|
Chemotherapy
|
46 |
46 |
Table 8 shows the study results. Disease-free survival was measured as the time from randomization to the earliest event of loco-regional or distant recurrence of the primary disease or development of contralateral breast cancer or death. DFS by hormone receptor status, nodal status and adjuvant chemotherapy were similar to the overall results. Data were premature for an analysis of survival.
Table 8: Extended Adjuvant Study Results
| Letrozole Tablets, USP N = 2582 |
Placebo N=2586 |
Hazard Ratio (95% Cl) | P-Value | |
|---|---|---|---|---|
|
Disease Free Survival (DFS)1 Events
|
122 (4.7%) |
193 (7.5%) |
0.62 (0.49, 0.78)2
|
0.00003 |
| Local Breast Recurrence |
9 |
22 |
|
|
| Local Chest Wall Recurrence |
2 |
8 |
|
|
| Regional Recurrence |
7 |
4 |
|
|
| Distant Recurrence |
55 |
92 |
0.61 (0.44 - 0.84) |
0.003 |
| Contralateral Breast Cancer |
19 |
29 |
|
|
| Deaths Without Recurrence or Contralateral Breast Cancer |
30 |
38 |
|
|
CI = confidence interval for hazard ratio. Hazard ratio of less than 1.0 indicates difference in favor of letrozole tablets, USP (lesser risk of recurrence); hazard ratio greater than 1.0 indicates difference in favor of placebo (higher risk of recurrence with letrozole tablets, USP).
P-value based on stratified logrank test.
1 First event of loco-regional recurrence, distant relapse, contralateral breast cancer or death from any cause
2 Analysis stratified by receptor status, nodal status and prior adjuvant chemotherapy (stratificationfactors as at randomization).
14.3 Updated Analyses of Extended Adjuvant Treatment of Early Breast Cancer, Median Treatment Duration of 60 Months
Table 9: Update of Extended Adjuvant Study Results
| Letrozole Tablets, USP N = 2582 |
Placebo N = 2586 |
Hazard Ratio (95% CI) |
P-Value |
|
|---|---|---|---|---|
Disease Free Survival (DFS) events |
344 (13.3) |
402 (15.5) |
0.89 (0.77, 1.03) |
0.12 |
|
Breast cancer recurrence
(Protocol definition of DFS events  |
209 |
286 |
0.75 (0.63, 0.89) |
0.001 |
| Local Breast Recurrence |
15 |
44 |
|
|
| Local Chest Wall Recurrence |
6 |
14 |
|
|
| Regional Recurrence |
10 |
8 |
|
|
| Distant Recurrence |
140 |
167 |
|
|
| Distant recurrence (first or subsequent events) |
142 |
169 |
0.88 (0.7,1.1) |
0.246 |
| Contralateral Breast Cancer |
37 |
53 |
|
|
| Deaths Without Recurrence or Contralateral Breast Cancer |
135 |
116 |
|
|
Updated analyses were conducted at a median follow-up of 62 months. In the letrozole tablets, USP arm, 71% of patients were treated for at least 3 years and 58% of patients completed at least 4.5 years of extended adjuvant treatment. After the unblinding of the study at a median follow-up of 28 months, approximately 60% of the selected patients in the placebo arm opted to switch to letrozole tablets, USP.
P
14.4 First-Line Treatment of Advanced Breast Cancer
A randomized, double-blind, multinational trial compared letrozole tablets, USP 2.5 mg with tamoxifen 20 mg in 916 postmenopausal patients with locally advanced (Stage IIIB or loco-regional recurrence not amenable to treatment with surgery or radiation) or metastatic breast cancer. Time to progression (TTP) was the primary endpoint of the trial. Selected baseline characteristics for this study are shown in Table 10.
Table 10: Selected Study Population Demographics
| Baseline Status | Letrozole Tablets, USP N=458 |
tamoxifen N=458 |
|---|---|---|
|
Stage of Disease
|
||
| IIIB |
6% |
7% |
| IV |
93% |
92% |
|
Receptor Status
|
||
| ER and PgR Positive |
38% |
41% |
| ER or PgR Positive |
26% |
26% |
| Both Unknown |
34% |
33% |
| ER-or PgR-/ Other Unknown |
<1% |
0 |
|
Previous Antiestrogen Therapy
|
||
| Adjuvant |
19% |
18% |
| None |
81% |
82% |
|
Dominant Site of Disease
|
||
| Soft Tissue |
25% |
25% |
| Bone |
32% |
29% |
| Viscera |
43% |
46% |
Letrozole tablets, USP was superior to tamoxifen in TTP and rate of objective tumor response (see Table 11).
Table 11 summarizes the results of the trial, with a total median follow-up of approximately 32 months. (All analyses are unadjusted and use 2-sided P-values.)
Table 11: Results of First-Line Treatment of Advanced Breast Cancer
| Letrozole Tablets, USP, 2.5 mg N=453 |
tamoxifen, 20 mg N=454 |
Hazard or Odds Ratio (95% CI) P-Value (2-Sided) |
|
|---|---|---|---|
|
Median Time to Progression
|
9.4 months |
6 months |
0.72 (0.62, 0.83) P<0.0001 |
|
Objective Response Rate
|
|||
| (CR + PR) |
145 (32%) |
95 (21%) |
1.77 (1.31, 2.39) P=0.0002 |
| (CR) |
42 (9%) |
15 (3%) |
2.99 (1.63, 5.47) P=0.0004 |
|
Duration of Objective Response
|
|||
| Median |
18 months (N=145) |
16 months (N=95) |
|
|
Overall Survival
|
35 months (N=458) |
32 months (N=458) |
P=0.5136 |
Figure 2 shows the Kaplan-Meier curves for TTP.
Figure 2 Kaplan-Meier Estimates of Time to Progression (Tamoxifen Study)

Table 12 shows results in the subgroup of women who had received prior antiestrogen adjuvant therapy, Table 13, results by disease site and Table 14, the results by receptor status.
Table 12: Efficacy in Patients Who Received Prior Antiestrogen Therapy
| Variable | Letrozole Tablets, USP 2.5 mg N=84 |
tamoxifen 20 mg N=83 |
|---|---|---|
|
Hazard ratio less than 1 or odds ratio greater than 1 favors letrozole tablets, USP; hazard ratio greater than 1 or odds ratio less than 1 favors tamoxifen |
||
|
Median Time to Progression(95% CI) |
8.9 months (6.2, 12.5) |
5.9 months (3.2, 6.2) |
| Hazard Ratio for TTP (95% CI) |
0.6 (0.43, 0.84) |
|
|
Objective Response Rate
|
||
| (CR + PR) |
22 (26%) |
7 (8%) |
| Odds Ratio for Response (95% CI) |
3.85 (1.5, 9.6) |
|
Table 13: Efficacy by Disease Site
| Letrozole Tablets, USP, 2.5 mg |
tamoxifen 20 mg |
|
|---|---|---|
|
Dominant Disease Site
|
||
|
Soft Tissue:
|
N=113 |
N=115 |
| Median TTP |
12.1 months |
6.4 months |
| Objective Response Rate |
50% |
34% |
|
Bone:
|
N=145 |
N=131 |
| Median TTP |
9.5 months |
6.3 months |
| Objective Response Rate |
23% |
15% |
|
Viscera:
|
N=195 |
N=208 |
| Median TTP |
8.3 months |
4.6 months |
| Objective Response Rate |
28% |
17% |
Table 14: Efficacy by Receptor Status
| Variable | Letrozole Tablets, USP 2.5 mg | tamoxifen 20 mg |
|---|---|---|
|
Hazard ratio less than 1 or odds ratio greater than 1 favors Letrozole tablet; hazard ratio greater than 1 or odds ratio less than 1 favors tamoxifen. |
||
|
Receptor Positive
|
N=294 |
N=305 |
| Median Time to Progression (95% CI) |
9.4 months (8.9, 11.8) |
6 months (5.1, 8.5) |
| Hazard Ratio for TTP (95% CI) |
0.69 (0.58, 0.83) |
|
| Objective Response Rate (CR+PR) |
97 (33%) |
66 (22%) |
| Odds Ratio for Response (95% CI) |
1.78 (1.2, 2.6) |
|
|
Receptor Unknown
|
N=159 |
N=149 |
| Median Time to Progression (95% CI) |
9.2 months (6.1, 12.3) |
6 months (4.1, 6.4) |
| Hazard Ratio for TTP (95% CI) |
0.77 (0.6, 0.99) |
|
| Objective Response Rate (CR+PR) |
48 (30%) |
29 (20%) |
| Odds Ratio for Response (95% CI) |
1.79 (1.1, 3) |
|
Figure 3 shows the Kaplan-Meier curves for survival.
Figure 3 Survival by Randomized Treatment Arm

Legend:Randomized letrozole tablets, USP: n=458, events 57%, median overall survival 35 months (95% CI 32 to 38 months)
Randomized tamoxifen: n=458, events 57%, median overall survival 32 months (95% CI 28 to 37 months)
Overall logrank P=0.5136 (i.e., there was no significant difference between treatment arms in overall survival).
P
14.5 Second-Line Treatment of Advanced Breast Cancer
Letrozole tablets, USP was initially studied at doses of 0.1 mg to 5 mg daily in six non-comparative Phase I/II trials in 181 postmenopausal estrogen/progesterone receptor positive or unknown advanced breast cancer patients previously treated with at least antiestrogen therapy. Patients had received other hormonal therapies and also may have received cytotoxic therapy. Eight (20%) of forty patients treated with letrozole tablets, USP 2.5 mg daily in Phase I/II trials achieved an objective tumor response (complete or partial response).
Two large randomized, controlled, multinational (predominantly European) trials were conducted in patients with advanced breast cancer who had progressed despite antiestrogen therapy. Patients were randomized to letrozole tablets, USP 0.5 mg daily, letrozole tablets, USP 2.5 mg daily, or a comparator (megestrol acetate 160 mg daily in one study; and aminoglutethimide 250 mg b.i.d. with corticosteroid supplementation in the other study). In each study over 60% of the patients had received therapeutic antiestrogens, and about one-fifth of these patients had had an objective response. The megestrol acetate controlled study was double-blind; the other study was open label. Selected baseline characteristics for each study are shown in Table 15.
Table 15: Selected Study Population Demographics
| Parameter | megestrol acetate study | aminoglutethimide study |
|---|---|---|
|
No. of Participants
|
552 |
557 |
|
Receptor Status
|
||
| ER/PR Positive |
57% |
56% |
| ER/PR Unknown |
43% |
44% |
|
Previous Therapy
|
||
| Adjuvant Only |
33% |
38% |
| Therapeutic +/- Adj. |
66% |
62% |
|
Sites of Disease
|
||
| Soft Tissue |
56% |
50% |
| Bone |
50% |
55% |
| Viscera |
40% |
44% |
Table 16 shows the results for the first trial, with a minimum follow-up of 15 months, that compared letrozole tablets, USP 0.5 mg, letrozole tablets, USP 2.5 mg, and megestrol acetate 160 mg daily. (All analyses are unadjusted.)
Table 16: Megestrol Acetate Study Results
| Letrozole Tablets, USP 0.5 mg N=188 |
Letrozole Tablets, USP 2.5 mg N=174 |
megestrol acetate N=190 |
|
|---|---|---|---|
|
Objective Response (CR + PR)
|
22 (11.7%) |
41 (23.6%) |
31 (16.3%) |
|
Median Duration of Response
|
552 days |
(Not reached) |
561 days |
|
Median Time to Progression
|
154 days |
170 days |
168 days |
|
Median Survival
|
633 days |
730 days |
659 days |
|
Odds Ratio for Response
|
Letrozole 2.5 mg :Letrozole 0.5 mg = 2.33 (95% CI: 1.32, 4.17); P=0.004  |
Letrozole 2.5 mg: megestrol = 1.58 (95% CI: 0.94, 2.66); P = 0.08  |
|
|
Relative Risk of Progression
|
Letrozole 2.5 mg : Letrozole0.5 mg = 0.81 (95% CI: 0.63, 1.03); P=0.09  |
Letrozole 2.5 mg : megestrol= 0.77 (95% CI: 0.6, 0.98), P=0.03  |
|
Figure 4 Kaplan-Meier Estimates of Time to Progression (Megestrol Acetate Study)
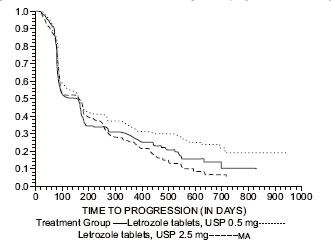
The results for the study comparing letrozole tablets, USP to aminoglutethimide, with a minimum follow-up of 9 months, are shown in Table 17. (Unadjusted analyses are used.)
Table 17:Aminoglutethimide Study Results
| Letrozole Tablets, USP 0.5 mg N=193 |
Letrozole Tablets, USP 2.5 mg N=185 |
aminoglutethimide N=179 |
|
|---|---|---|---|
|
Objective Response (CR + PR)
|
34 (17.6%) |
34 (18.4%) |
22 (12.3%) |
|
Median Duration of Response
|
619 days |
706 days |
450 days |
|
Median Time to Progression
|
103 days |
123 days |
112 days |
|
Median Survival
|
636 days |
792 days |
592 days |
|
Odds Ratio for Response
|
Letrozole 2.5 mg : Letrozole 0.5 mg = 1.05 (95% CI: 0.62, 1.79); P=0.85  |
Letrozole 2.5 mg : aminoglutethimide=1.61 (95% CI: 0.9, 2.87); P=0.11  |
|
|
Relative Risk of Progression
|
Letrozole 2.5 mg : Letrozole 0.5 mg =0.86 (95% CI: 0.68, 1.11); P=0.25  |
Letrozole 2.5 mg : aminoglutethimide = 0.74 (95% CI: 0.57, 0.94); P=0.02  |
|
The Kaplan-Meier curves for progression for the aminoglutethimide study is shown in Figure 5.
Figure 5 Kaplan-Meier Estimates of Time to Progression (Aminoglutethimide Study)

16 HOW SUPPLIED/STORAGE AND HANDLING
|
Product No. |
NDC No. |
Strength
|
|
| 772030 |
63323-772-30 |
2.5 mg |
HDPE bottle containing 30 tablets |
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Information for Patients
Pregnancy: Letrozole tablets, USP is contraindicated in women of premenopausal endocrine status. The physician needs to discuss the necessity of adequate contraception with women who have the potential to become pregnant including women who are perimenopausal or who recently became postmenopausal, until their postmenopausal status is fully established.
Fatigue and Dizziness: Since fatigue and dizziness have been observed with the use of letrozole tablets, USP and somnolence was uncommonly reported, caution is advised when driving or using machinery.

APP Pharmaceuticals, LLC
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

LETROZOLE
TABLETS, USP
30 Tablets
LETROZOLELETROZOLE TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||